Journal of Controlled Release ( IF 10.5 ) Pub Date : 2021-10-20 , DOI: 10.1016/j.jconrel.2021.10.021 Linying Li 1 , Gregory J Gatto 2 , Rhonda M Brand 3 , Sai Archana Krovi 1 , Mackenzie L Cottrell 4 , Chasity Norton 1 , Ariane van der Straten 5 , Leah M Johnson 1
|
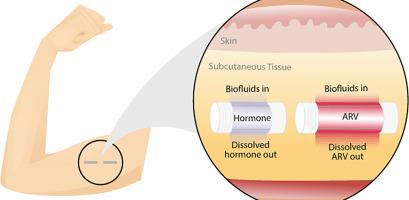
Women worldwide confront two major reproductive health challenges: the need for contraception and protection from sexually transmitted infections, including Human Immunodeficiency Virus (HIV). Multipurpose Prevention Technologies (MPTs) that simultaneously prevent unintended pregnancy and HIV could address these challenges with a single product. Here, we developed a long-acting (LA) subcutaneously administered and biodegradable implant system that provides sustained delivery of contraceptive and antiretroviral (ARV) with zero-order release kinetics. The MPT system involves two implants comprising an extruded tube of a biodegradable polymer, poly(ε-caprolactone) (PCL). Each implant is filled with a formulation of progestin [levonorgestrel (LNG) or etonogestrel (ENG)], or a formulation of a potent ARV [tenofovir alafenamide (TAF), or 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA)]. We demonstrated sustained in-vitro release of LNG, ENG, and EFdA from the implant system for 13–17 months, while maintaining high stability of the drugs (>99%) within the implant reservoirs. We further elucidated the controlled release mechanism of the implant and leveraged several tunable parameters (e.g., type and quantity of the excipient, PCL properties, and implant wall thickness) to tailor the release kinetics and enhance the mechanical integrity of the MPT implant. The optimized MPT showed sustained in-vitro release of ENG and EFdA over 1 year while maintaining a high level of formulation stability and structural integrity. The MPT implant system was further evaluated in a preclinical study using a rodent model and demonstrated sustained release of EFdA (6 months) and ENG (12 months) with high stability of the drug formulation (>95%). This manuscript supports the continued advancement of LA delivery systems for MPTs.
中文翻译:

用于持续递送抗逆转录病毒药物 (ARV) 和激素的长效可生物降解植入物
世界各地的妇女面临两大生殖健康挑战:避孕和防止性传播感染,包括人类免疫缺陷病毒 (HIV)。同时预防意外怀孕和 HIV 的多用途预防技术 (MPT) 可以通过单一产品解决这些挑战。在这里,我们开发了一种长效 (LA) 皮下给药和可生物降解的植入系统,可提供具有零级释放动力学的避孕和抗逆转录病毒 (ARV) 的持续递送。MPT 系统包括两个植入物,包括一个由可生物降解的聚合物聚(ε-己内酯)(PCL)制成的挤压管。每个植入物都填充有孕激素制剂 [左炔诺孕酮 (LNG) 或依托孕烯 (ENG)],或强效 ARV 制剂 [替诺福韦艾拉酚胺 (TAF),或 4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA)]。我们证明了 LNG、ENG 和 EFdA 从植入系统体外持续释放 13-17 个月,同时保持植入物储库内药物的高稳定性 (>99%)。我们进一步阐明了植入物的控释机制,并利用几个可调参数(例如,赋形剂的类型和数量、PCL 特性和植入物壁厚)来调整释放动力学并增强 MPT 植入物的机械完整性。优化的 MPT 显示 ENG 和 EFdA 在体外持续释放超过 1 年,同时保持高水平的配方稳定性和结构完整性。在使用啮齿动物模型的临床前研究中进一步评估了 MPT 植入系统,并证明了 EFdA(6 个月)和 ENG(12 个月)的持续释放以及药物配方的高稳定性(>95%)。这份手稿支持 MPT 的 LA 交付系统的持续发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号