当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Inverse-Electron-Demand Diels–Alder Reactions of 2-Pyrones with Indenes: Total Syntheses of Cephanolides A and B
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-10-20 , DOI: 10.1002/anie.202112223 Yang Lu 1 , Meng-Meng Xu 1 , Zhi-Mao Zhang 1 , Junliang Zhang 1 , Quan Cai 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-10-20 , DOI: 10.1002/anie.202112223 Yang Lu 1 , Meng-Meng Xu 1 , Zhi-Mao Zhang 1 , Junliang Zhang 1 , Quan Cai 1
Affiliation
|
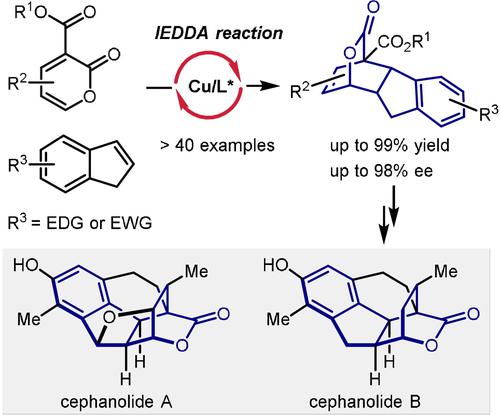
Catalytic asymmetric all-carbon-based inverse-electron-demand Diels–Alder reactions of 2-pyrones with electronically unbiased indenes have been realized. This method enables the rapid and enantioselective construction of a wide range of hexahydrofluorenyl bridged-lactone scaffolds. Based on this method, asymmetric total syntheses of cephanolides A and B have been accomplished.

中文翻译:

2-吡喃酮与茚的催化不对称逆电子需量 Diels-Alder 反应:头孢内酯 A 和 B 的全合成
2-吡喃酮与电子无偏茚的催化不对称全碳基逆电子需求 Diels-Alder 反应已经实现。这种方法能够快速和对映选择性地构建范围广泛的六氢芴基桥接内酯支架。基于该方法,完成了头孢内酯A和B的不对称全合成。

更新日期:2021-12-06

中文翻译:

2-吡喃酮与茚的催化不对称逆电子需量 Diels-Alder 反应:头孢内酯 A 和 B 的全合成
2-吡喃酮与电子无偏茚的催化不对称全碳基逆电子需求 Diels-Alder 反应已经实现。这种方法能够快速和对映选择性地构建范围广泛的六氢芴基桥接内酯支架。基于该方法,完成了头孢内酯A和B的不对称全合成。












































 京公网安备 11010802027423号
京公网安备 11010802027423号