European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-10-19 , DOI: 10.1016/j.ejmech.2021.113928 Bing Zhang 1 , Yulin Duan 1 , Yuwei Yang 1 , Qing Mao 1 , Fengwei Lin 1 , Jun Gao 1 , Xiwen Dai 1 , Peng Zhang 1 , Qiuhua Li 1 , Jinxin Li 1 , Ronghua Dai 2 , Shaojie Wang 1
|
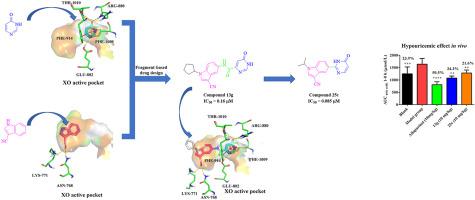
Xanthine oxidase (XO) has been an important target for the treatment of hyperuricemia and gout. The analysis of potential interactions of pyrimidinone and 3-cyano indole pharmacophores present in the corresponding reported XO inhibitors with parts of the XO active pocket indicated that they both can be used as effective fragments for the fragment-based design of nonpurine XO inhibitors. In this paper, we adopted the fragment-based drug design strategy to link the two fragments with an amide bond to design the type 1 compounds 13a–13w,14c, 14d, 14f, 14g, 14j, 14k, and 15g. Compound 13g displayed an evident XO inhibitory potency (IC50 = 0.16 μM), which was 52.3-fold higher than that of allopurinol (IC50 = 8.37 μM). For comparison, type 2 compounds 5-(6-oxo-1,6-dihydropyrimidin-2-yl)-1H-indole-3-carbonitriles (25c–25g) were also designed by linking the two fragments with a single bond directly. The results showed that compound 25c from the latter series displayed the best inhibitory potency (IC50 = 0.085 μM), and it was 98.5-fold stronger than that of allopurinol (IC50 = 8.37 μM). These results suggested that amide and single bonds were applicable for linking the two fragments together to obtain potent nonpurine XO inhibitors. The structure–activity relationship results revealed that hydrophobic groups at N-atom of the indole moiety were indispensable for the improvement of the inhibitory potency in vitro against XO. In addition, enzyme kinetics studies suggested that compounds 13g and 25c, as the most promising XO inhibitors for the two types of target compounds, acted as mixed-type inhibitors for XO. Moreover, molecular modeling studies suggested that the pyrimidinone and indole moieties of the target compounds could interact well with key amino acid residues in the active pocket of XO. Furthermore, in vivo hypouricemic effect demonstrated that compounds 13g and 25c could effectively reduce serum uric acid levels at an oral dose of 10 mg/kg. Therefore, compounds 13g and 25c could be potential and efficacious agents for the treatment of hyperuricemia and gout.
中文翻译:

N-(3-cyano-1H-indol-5/6-yl)-6-oxo-1,6-dihydropyrimidine-4-carboxamides 和 5-(6-oxo-1,6) 的设计、合成和生物学评价-dihydropyrimidin-2-yl)-1H-indole-3-carbonitriles 作为新型黄嘌呤氧化酶抑制剂
黄嘌呤氧化酶(XO)一直是治疗高尿酸血症和痛风的重要靶点。对相应报道的 XO 抑制剂中存在的嘧啶酮和 3-氰基吲哚药效团与部分 XO 活性口袋的潜在相互作用的分析表明,它们都可以用作基于片段的非嘌呤 XO 抑制剂设计的有效片段。在本文中,我们采用基于片段的药物设计策略,通过酰胺键将两个片段连接起来,设计出 1 型化合物13a–13w、14c、14d、14f、14g、14j、14k和15g。复方13g显示出明显的 XO 抑制效力 (IC 50 = 0.16 μM),比别嘌醇 (IC 50 = 8.37 μM) 高 52.3 倍。为了比较,还设计了 2 型化合物 5-(6-oxo-1,6-dihydropyrimidin-2-yl)-1 H -indole-3-carbonitriles ( 25c – 25g ) 通过直接用单键连接两个片段. 结果表明,后者系列的化合物25c显示出最好的抑制效力(IC 50 = 0.085 μM),比别嘌醇(IC 50 )强 98.5 倍。 = 8.37 微米)。这些结果表明,酰胺和单键可用于将两个片段连接在一起以获得有效的非嘌呤 XO 抑制剂。构效关系结果表明,吲哚部分N原子上的疏水基团对于提高体外对XO的抑制效力是必不可少的。此外,酶动力学研究表明化合物13g和25c,作为两种目标化合物最有希望的XO抑制剂,作为XO的混合型抑制剂。此外,分子模型研究表明,目标化合物的嘧啶酮和吲哚部分可以与 XO 活性口袋中的关键氨基酸残基良好相互作用。此外,体内降尿酸作用表明,化合物13g和25c在口服剂量为 10 mg/kg 时可有效降低血清尿酸水平。因此,化合物13g和25c可能是治疗高尿酸血症和痛风的潜在有效药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号