当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic resolution of azaflavanones via a RuPHOX-Ru catalyzed asymmetric hydrogenation
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-08 , DOI: 10.1039/d1qo01310f Yue Zhu 1 , Jiayu Zhou 1 , Jing Li 1 , Kai Xu 2 , Jianxun Ye 1 , Yufei Lu 1 , Delong Liu 1 , Wanbin Zhang 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-10-08 , DOI: 10.1039/d1qo01310f Yue Zhu 1 , Jiayu Zhou 1 , Jing Li 1 , Kai Xu 2 , Jianxun Ye 1 , Yufei Lu 1 , Delong Liu 1 , Wanbin Zhang 1, 2
Affiliation
|
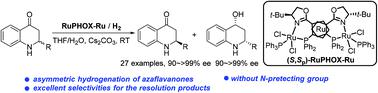
The kinetic resolution of azaflavanones has been established via RuPHOX-Ru catalyzed asymmetric hydrogenation, providing chiral azaflavanones and azaflavanols in high yields with up to >20 : 1 dr and 99.7% ee. The highly efficient kinetic resolution process has been illustrated based on the deuterium labelling and racemization experiments. The reaction could be performed on a gram-scale with a relatively low catalyst loading (2000 S/C), and the resulting products allow for several transformations.
中文翻译:

通过 RuPHOX-Ru 催化的不对称氢化对氮杂黄烷酮进行动力学拆分
氮杂黄烷酮的动力学拆分是通过RuPHOX-Ru 催化的不对称氢化建立的,以高产率提供手性氮杂黄烷酮和氮杂黄烷醇,高达 >20:1 dr 和 99.7% ee。基于氘标记和外消旋化实验说明了高效的动力学拆分过程。该反应可以在克级规模上进行,催化剂负载量相对较低 (2000 S/C),所得产物允许进行多次转化。
更新日期:2021-10-19
中文翻译:

通过 RuPHOX-Ru 催化的不对称氢化对氮杂黄烷酮进行动力学拆分
氮杂黄烷酮的动力学拆分是通过RuPHOX-Ru 催化的不对称氢化建立的,以高产率提供手性氮杂黄烷酮和氮杂黄烷醇,高达 >20:1 dr 和 99.7% ee。基于氘标记和外消旋化实验说明了高效的动力学拆分过程。该反应可以在克级规模上进行,催化剂负载量相对较低 (2000 S/C),所得产物允许进行多次转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号