当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Flexibility Enhances Reactivity: Redox Isomerism and Jahn–Teller Effects in a Bioinspired Mn4O4 Cubane Water Oxidation Catalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-18 , DOI: 10.1021/acscatal.1c03566 Ludwig Schwiedrzik 1 , Vera Brieskorn 1 , Leticia González 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-18 , DOI: 10.1021/acscatal.1c03566 Ludwig Schwiedrzik 1 , Vera Brieskorn 1 , Leticia González 1
Affiliation
|
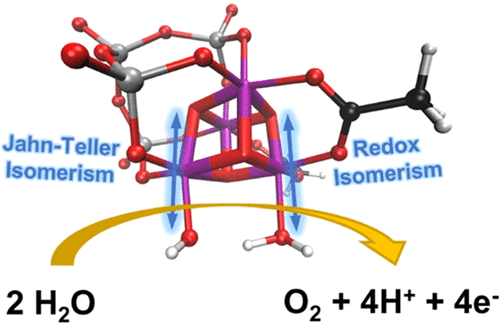
Understanding how water oxidation to molecular oxygen proceeds in molecular metal-oxo catalysts is a challenging endeavor due to their structural complexity. In this report, we unravel the water oxidation mechanism of the highly active water oxidation catalyst [Mn4V4O17(OAc)3]3–, a polyoxometalate catalyst with a [Mn4O4]6+ cubane core reminiscent of the natural oxygen-evolving complex. Starting from the activated species [Mn44+V4O17(OAc)2(H2O)(OH)]1–, we scrutinized multiple pathways to find that water oxidation proceeds via a sequential proton-coupled electron transfer (PCET), O–O bond formation, another PCET, an intramolecular electron transfer, and another PCET resulting in O2 evolution, with a predicted thermodynamic overpotential of 0.71 V. An in-depth investigation of the O–O bond formation process revealed an essential interplay between redox isomerism and Jahn–Teller effects, responsible for enhancing reactivity in the catalytic cycle. This is achieved by redistributing electrons between metal centers and weakening relevant bonds through Jahn–Teller distortions, introducing flexibility to the otherwise rigid cubane core of the catalyst. These mechanistic insights are expected to advance the design of efficient bioinspired Mn cubane water-splitting catalysts.
中文翻译:

灵活性增强反应性:仿生 Mn4O4 古巴烷水氧化催化剂中的氧化还原异构现象和 Jahn-Teller 效应
由于其结构的复杂性,了解分子金属氧催化剂中水氧化成分子氧的过程是一项具有挑战性的工作。在本报告中,我们揭示了高活性水氧化催化剂 [Mn 4 V 4 O 17 (OAc) 3 ] 3–的水氧化机理,这是一种具有 [Mn 4 O 4 ] 6+立方烷核心的多金属氧酸盐催化剂,让人想起天然放氧复合物。从活化物质 [Mn 4 4+ V 4 O 17 (OAc) 2 (H 2 O)(OH)] 1–开始,我们仔细研究了多种途径,发现水氧化是通过连续的质子耦合电子转移(PCET)进行的。 ),O-O 键形成,另一个 PCET,分子内电子转移,另一个 PCET 导致 O 2演化,预测热力学超电势为 0.71 V。对 O-O 键形成过程的深入研究揭示了一个重要的过程氧化还原异构和 Jahn-Teller 效应之间的相互作用,负责增强催化循环中的反应性。这是通过在金属中心之间重新分配电子并通过 Jahn-Teller 畸变削弱相关键来实现的,从而为催化剂的刚性立方烷核心引入灵活性。这些机理见解有望推进高效仿生立方锰锰水分解催化剂的设计。
更新日期:2021-11-05
中文翻译:

灵活性增强反应性:仿生 Mn4O4 古巴烷水氧化催化剂中的氧化还原异构现象和 Jahn-Teller 效应
由于其结构的复杂性,了解分子金属氧催化剂中水氧化成分子氧的过程是一项具有挑战性的工作。在本报告中,我们揭示了高活性水氧化催化剂 [Mn 4 V 4 O 17 (OAc) 3 ] 3–的水氧化机理,这是一种具有 [Mn 4 O 4 ] 6+立方烷核心的多金属氧酸盐催化剂,让人想起天然放氧复合物。从活化物质 [Mn 4 4+ V 4 O 17 (OAc) 2 (H 2 O)(OH)] 1–开始,我们仔细研究了多种途径,发现水氧化是通过连续的质子耦合电子转移(PCET)进行的。 ),O-O 键形成,另一个 PCET,分子内电子转移,另一个 PCET 导致 O 2演化,预测热力学超电势为 0.71 V。对 O-O 键形成过程的深入研究揭示了一个重要的过程氧化还原异构和 Jahn-Teller 效应之间的相互作用,负责增强催化循环中的反应性。这是通过在金属中心之间重新分配电子并通过 Jahn-Teller 畸变削弱相关键来实现的,从而为催化剂的刚性立方烷核心引入灵活性。这些机理见解有望推进高效仿生立方锰锰水分解催化剂的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号