Molecular Therapy ( IF 12.1 ) Pub Date : 2021-10-19 , DOI: 10.1016/j.ymthe.2021.10.011 Andreas Hombach 1 , Markus Barden 2 , Lisa Hannappel 3 , Markus Chmielewski 1 , Gunter Rappl 3 , Agapios Sachinidis 4 , Hinrich Abken 2
|
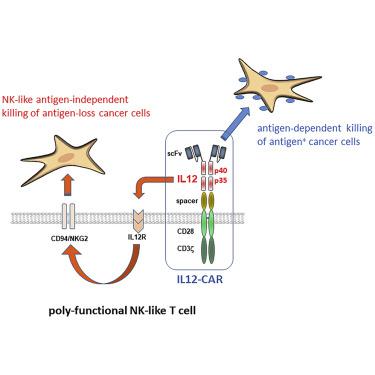
Chimeric antigen receptor (CAR)-redirected T cell therapy often fails to control tumors in the long term due to selecting cancer cells that downregulated or lost CAR targeted antigen. To reprogram the functional capacities specifically of engineered CAR T cells, we inserted IL12 into the extracellular moiety of a CD28-ζ CAR; both the CAR endodomain and IL12 were functionally active, as indicated by antigen-redirected effector functions and STAT4 phosphorylation, respectively. The IL12-CAR reprogrammed CD8+ T cells toward a so far not recognized natural killer (NK) cell-like signature and a CD94+CD56+CD62Lhigh phenotype closely similar, but not identical, to NK and cytokine induced killer (CIK) cells. In contrast to conventional CAR T cells, IL12-CAR T cells acquired antigen-independent, human leukocyte antigen E (HLA-E) restricted cytotoxic capacities eliminating antigen-negative cancer cells in addition to eliminating cancer cells with CAR cognate antigen. Simultaneous signaling through both the CAR endodomain and IL12 were required for inducing maximal NK-like cytotoxicity; adding IL12 to conventional CAR T cells was not sufficient. Antigen-negative tumors were attacked by IL12-CAR T cells, but not by conventional CAR T cells. Overall, we present a prototype of a new family of CARs that augments tumor recognition and elimination through expanded functional capacities by an appropriate cytokine integrated into the CAR exodomain.
中文翻译:

整合到 CAR 外域的 IL12 将 CD8+ T 细胞转化为多功能 NK 样细胞,对抗原缺失肿瘤具有出色的杀伤力
由于选择下调或丢失 CAR 靶向抗原的癌细胞,嵌合抗原受体 (CAR) 重定向的 T 细胞疗法通常无法长期控制肿瘤。为了重新编程工程化 CAR T 细胞的功能能力,我们将 IL12 插入到 CD28-ζ CAR 的细胞外部分;CAR 内域和 IL12 都具有功能活性,分别由抗原重定向效应子功能和 STAT4 磷酸化表明。IL12-CAR 将 CD8 + T 细胞重新编程为迄今为止尚未识别的自然杀伤 (NK) 细胞样特征和 CD94 + CD56 + CD62L高表型与 NK 和细胞因子诱导的杀伤 (CIK) 细胞非常相似,但不完全相同。与传统的 CAR T 细胞相比,IL12-CAR T 细胞获得了抗原非依赖性、人类白细胞抗原 E (HLA-E) 限制性细胞毒性能力,除了消除具有 CAR 同源抗原的癌细胞外,还能消除抗原阴性的癌细胞。诱导最大的 NK 样细胞毒性需要通过 CAR 内域和 IL12 同时发出信号;向传统 CAR T 细胞中添加 IL12 是不够的。抗原阴性肿瘤受到 IL12-CAR T 细胞的攻击,但不受常规 CAR T 细胞的攻击。总的来说,我们展示了一个新的 CAR 家族的原型,它通过将适当的细胞因子整合到 CAR 外域中来扩展功能能力来增强肿瘤识别和消除。











































 京公网安备 11010802027423号
京公网安备 11010802027423号