当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Insights into the Human Mitochondrial Pyruvate Carrier Complexes
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-10-19 , DOI: 10.1021/acs.jcim.1c00879 Liang Xu 1 , Clyde F Phelix 2 , Liao Y Chen 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-10-19 , DOI: 10.1021/acs.jcim.1c00879 Liang Xu 1 , Clyde F Phelix 2 , Liao Y Chen 1
Affiliation
|
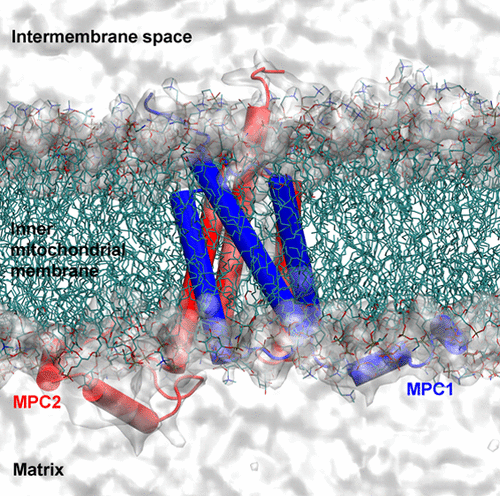
Pyruvate metabolism requires the mitochondrial pyruvate carrier (MPC) proteins to transport pyruvate from the intermembrane space through the inner mitochondrial membrane to the mitochondrial matrix. The lack of the atomic structures of MPC hampers the understanding of the functional states of MPC and molecular interactions with the substrate or inhibitor. Here, we develop the de novo models of human MPC complexes and characterize the conformational dynamics of the MPC heterodimer formed by MPC1 and MPC2 (MPC1/2) by computational simulations. Our results reveal that functional MPC1/2 prefers to adopt an inward-open conformation, with the carrier open to the matrix side, whereas the outward-open states are less populated. The energy barrier for pyruvate transport in MPC1/2 is low enough, and the inhibitor UK5099 blocks the pyruvate transport by stably binding to MPC1/2. Notably, consistent with experimental results, the MPC1 L79H mutation significantly alters the conformations of MPC1/2 and thus fails for substrate transport. However, the MPC1 R97W mutation seems to retain the transport activity. The present de novo models of MPC complexes provide structural insights into the conformational states of MPC complexes and mechanistic understanding of interactions between the substrate/inhibitor and MPC proteins.
中文翻译:

对人体线粒体丙酮酸载体复合物的结构洞察
丙酮酸代谢需要线粒体丙酮酸载体 (MPC) 蛋白将丙酮酸从膜间隙通过线粒体内膜转运至线粒体基质。MPC 原子结构的缺乏阻碍了对 MPC 的功能状态以及与底物或抑制剂的分子相互作用的理解。在这里,我们开发了人类 MPC 复合物的从头模型,并通过计算模拟表征了由 MPC1 和 MPC2 (MPC1/2) 形成的 MPC 异二聚体的构象动力学。我们的结果表明,功能性 MPC1/2 更喜欢采用向内开放的构象,载体向矩阵侧开放,而向外开放的状态人口较少。MPC1/2 中丙酮酸转运的能垒足够低,抑制剂 UK5099 通过稳定结合 MPC1/2 来阻断丙酮酸转运。值得注意的是,与实验结果一致,MPC1 L79H 突变显着改变了 MPC1/2 的构象,因此底物转运失败。然而,MPC1 R97W 突变似乎保留了运输活动。目前的 MPC 复合物从头模型提供了对 MPC 复合物构象状态的结构见解以及对底物/抑制剂与 MPC 蛋白之间相互作用的机制理解。
更新日期:2021-11-22
中文翻译:

对人体线粒体丙酮酸载体复合物的结构洞察
丙酮酸代谢需要线粒体丙酮酸载体 (MPC) 蛋白将丙酮酸从膜间隙通过线粒体内膜转运至线粒体基质。MPC 原子结构的缺乏阻碍了对 MPC 的功能状态以及与底物或抑制剂的分子相互作用的理解。在这里,我们开发了人类 MPC 复合物的从头模型,并通过计算模拟表征了由 MPC1 和 MPC2 (MPC1/2) 形成的 MPC 异二聚体的构象动力学。我们的结果表明,功能性 MPC1/2 更喜欢采用向内开放的构象,载体向矩阵侧开放,而向外开放的状态人口较少。MPC1/2 中丙酮酸转运的能垒足够低,抑制剂 UK5099 通过稳定结合 MPC1/2 来阻断丙酮酸转运。值得注意的是,与实验结果一致,MPC1 L79H 突变显着改变了 MPC1/2 的构象,因此底物转运失败。然而,MPC1 R97W 突变似乎保留了运输活动。目前的 MPC 复合物从头模型提供了对 MPC 复合物构象状态的结构见解以及对底物/抑制剂与 MPC 蛋白之间相互作用的机制理解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号