当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Mechanism of Phosphate Steering for DNA Binding, Cleavage Localization, and Substrate Release in Nucleases
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-15 , DOI: 10.1021/acscatal.1c03346 Elisa Donati 1 , Pietro Vidossich 1 , Marco De Vivo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-15 , DOI: 10.1021/acscatal.1c03346 Elisa Donati 1 , Pietro Vidossich 1 , Marco De Vivo 1
Affiliation
|
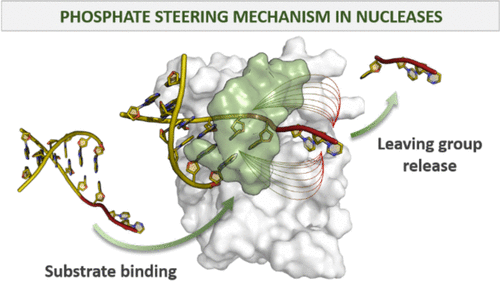
Structure-specific endonucleases (SSEs) cleave the DNA substrate in a precise position based on the specific DNA 3D structure. Human flap endonuclease 1 (hFEN1) is a 5′ SSE that prevents DNA instability by processing Okazaki fragment 5′-flaps with remarkable efficiency and selectivity using two-metal-ion catalysis. Recent structural and mutagenesis data of hFEN1 suggest that phosphate steering favors specificity and catalysis. Here, we investigate the phosphate steering mechanism at the atomistic level using microsecond-long molecular dynamics and well-tempered metadynamics simulations of wild-type and mutant systems of hFEN1. We show how positively charged second and third-shell residues operate the phosphate steering mechanism to promote catalysis through (i) substrate recruitment; (ii) precise cleavage localization; and (iii) substrate release, thus actively preventing the off-target incision of the substrate. Importantly, structural comparisons of hFEN1 and other nuclease enzymes suggest that phosphate steering may also serve the structure-based selection of the specific DNA substrate by other 5′ structure-specific nucleases.
中文翻译:

核酸酶中 DNA 结合、切割定位和底物释放的磷酸盐导向的分子机制
结构特异性核酸内切酶 (SSE) 根据特定的 DNA 3D 结构在精确位置切割 DNA 底物。人皮瓣核酸内切酶 1 (hFEN1) 是一种 5' SSE,它通过使用双金属离子催化以显着的效率和选择性处理冈崎片段 5'-皮瓣来防止 DNA 不稳定。hFEN1 的最新结构和诱变数据表明磷酸盐导向有利于特异性和催化作用。在这里,我们使用微秒长的分子动力学和 hFEN1 野生型和突变系统的良好元动力学模拟在原子水平上研究磷酸盐转向机制。我们展示了带正电荷的第二和第三壳残基如何操作磷酸盐导向机制以通过 (i) 底物募集促进催化作用;(ii) 精确的切割定位;(iii) 底物释放,从而主动防止基材的脱靶切口。重要的是,hFEN1 和其他核酸酶的结构比较表明,磷酸盐导向也可以用于其他 5' 结构特异性核酸酶对特定 DNA 底物的基于结构的选择。
更新日期:2021-11-05
中文翻译:

核酸酶中 DNA 结合、切割定位和底物释放的磷酸盐导向的分子机制
结构特异性核酸内切酶 (SSE) 根据特定的 DNA 3D 结构在精确位置切割 DNA 底物。人皮瓣核酸内切酶 1 (hFEN1) 是一种 5' SSE,它通过使用双金属离子催化以显着的效率和选择性处理冈崎片段 5'-皮瓣来防止 DNA 不稳定。hFEN1 的最新结构和诱变数据表明磷酸盐导向有利于特异性和催化作用。在这里,我们使用微秒长的分子动力学和 hFEN1 野生型和突变系统的良好元动力学模拟在原子水平上研究磷酸盐转向机制。我们展示了带正电荷的第二和第三壳残基如何操作磷酸盐导向机制以通过 (i) 底物募集促进催化作用;(ii) 精确的切割定位;(iii) 底物释放,从而主动防止基材的脱靶切口。重要的是,hFEN1 和其他核酸酶的结构比较表明,磷酸盐导向也可以用于其他 5' 结构特异性核酸酶对特定 DNA 底物的基于结构的选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号