Journal of Controlled Release ( IF 10.5 ) Pub Date : 2021-10-16 , DOI: 10.1016/j.jconrel.2021.10.017 Ella Peled 1 , Alejandro Sosnik 1
|
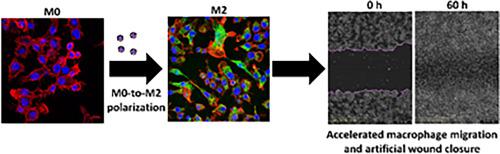
Macrophages are highly plastic phagocytic cells that can exist in distinct phenotypes and play key roles in physiological and pathological pathways. They can be classically activated to the pro-inflammatory M1 phenotype or alternatively activated to an M2 anti-inflammatory one by various stimuli in the biological milieu. Different biomaterials polarize macrophages to M1 or M2 phenotypes and emerged as a very promising strategy to modulate their activation and performance. In this work, we investigate the ability of drug-free amphiphilic nanoparticles (hydrodynamic diameter of ~130 nm) produced by the self-assembly of a graft copolymer of hydrolyzed galactomannan, a natural polysaccharide of galactose and mannose, that was hydrophobized in the side-chain with poly(methyl methacrylate) blocks and that can encapsulate hydrophobic drugs, to trigger macrophage polarization. The compatibility and uptake of the nanoparticles are demonstrated in the murine macrophage cell line RAW264.7 by a metabolic assay, confocal laser scanning fluorescence microscopy (CLSFM) and imaging flow cytometry in the absence and the presence of endocytosis inhibitors. Results indicate that they are internalized by both clathrin- and caveolin-mediated endocytosis. The ability of these drug-free nanoparticles to polarize these cells to the M2-like phenotype and to switch an M1 to an M2 phenotype is confirmed by the downregulation of the M1 marker cluster of differentiation 80 (CD80), and upregulation of M2 markers CD163 and CD206, as measured by flow cytometry and CLSFM. In addition, we preliminarily assess the effect of the nanoparticles on wound healing by tracking the closure of an artificial wound of RAW264.7 macrophages in a scratch assay. Findings indicate a faster closure of the wound in the presence of the nanoparticles with respect to untreated cells. Finally, a migration assay utilizing a macrophage/fibroblast co-culture model in vitro demonstrates that M2 polarization increases fibroblast migration by 24-fold with respect to untreated cells. These findings demonstrate the ability of this nanotechnology platform to interfere and change the macrophages phenotype in vitro and represent robust evidence for the investigation of their therapeutic performance alone or in combination with an encapsulated hydrophobic drug in wound models in vivo
中文翻译:

两亲性半乳甘露聚糖纳米粒子触发小鼠巨噬细胞的替代激活
巨噬细胞是高度可塑性的吞噬细胞,可以以不同的表型存在,并在生理和病理途径中发挥关键作用。它们可以通过生物环境中的各种刺激被经典地激活为促炎 M1 表型,或者被激活为 M2 抗炎表型。不同的生物材料将巨噬细胞极化为 M1 或 M2 表型,并成为调节其活化和性能的非常有前途的策略。在这项工作中,我们研究了由水解半乳甘露聚糖(半乳糖和甘露糖的天然多糖)的接枝共聚物自组装产生的无药物两亲纳米粒子(流体动力学直径约为 130 nm)的能力,该纳米粒子在侧面疏水化-带有聚(甲基丙烯酸甲酯)嵌段的链,可以封装疏水性药物,触发巨噬细胞极化。在小鼠巨噬细胞系 RAW264.7 中,通过代谢测定、共聚焦激光扫描荧光显微镜 (CLSFM) 和成像流式细胞术在不存在和存在内吞抑制剂的情况下证明了纳米颗粒的相容性和吸收。结果表明它们被网格蛋白和小窝蛋白介导的内吞作用内化。这些不含药物的纳米粒子将这些细胞极化为 M2 样表型并将 M1 转换为 M2 表型的能力通过 M1 分化标记簇 80 (CD80) 的下调和 M2 标记 CD163 的上调得到证实和 CD206,通过流式细胞术和 CLSFM 测量。此外,我们通过在划痕试验中跟踪 RAW264.7 巨噬细胞人工伤口的闭合情况,初步评估了纳米颗粒对伤口愈合的影响。研究结果表明,相对于未处理的细胞,在纳米颗粒存在的情况下,伤口闭合速度更快。最后,利用巨噬细胞/成纤维细胞共培养模型的迁移测定体外证明相对于未处理的细胞,M2 极化使成纤维细胞迁移增加了 24 倍。这些发现证明了该纳米技术平台在体外干扰和改变巨噬细胞表型的能力,并代表了在体内伤口模型中单独或与封装的疏水药物联合研究其治疗性能的有力证据











































 京公网安备 11010802027423号
京公网安备 11010802027423号