Journal of Controlled Release ( IF 10.5 ) Pub Date : 2021-10-16 , DOI: 10.1016/j.jconrel.2021.10.015 Abd Al-Wali Mohammed M Japir 1 , Wendong Ke 1 , Junjie Li 2 , Jean Felix Mukerabigwi 1 , Alhadi Ibrahim 1 , Yuheng Wang 1 , Xiang Li 1 , Qinghao Zhou 1 , Fathelrahman Mohammed 1 , Zhishen Ge 1
|
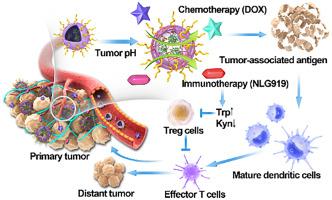
Combination chemo-immunotherapy of cancers has attracted great attention due to its significant synergistic antitumor effect. The response rates and therapeutic efficacy of immunotherapy can be enhanced significantly after proper combination with chemotherapy. However, chemo-immunotherapy is frequently limited by severe immune-related adverse events and systemic side toxicity. In this report, efficient nanofactory-directed enzyme prodrug chemo-immunotherapy is demonstrated based on enzyme-loaded tumor-dilatable polymersomes with optimized membrane cross-linking density. Upon intravenous injection of the nanofactories, they can passively accumulate at the tumor site. The tumor pH-responsive nanofactories can swell from ~100 nm to ~200 nm under the trigger of tumor acidity, leading to prolonged retention of up to one week inside tumor tissues. Simultaneously, the membrane permeability of the nanofactories has improved significantly, which allows hydrophilic small molecules to pass across the membranes while keeping the enzymes in the inner cavities. Subsequently, the non-toxic prodrug mixtures of chemo-immunotherapy are administrated three times within 6 days, which are in situ activated by the nanofactories selectively at tumor sites. Activated chemotherapeutic drugs kill cancer cells and generate tumor-associated antigens to promote the maturation of dendritic cells. Activated indoleamine 2, 3-dioxygenase 1 inhibitors reverse the immunosuppressive tumor microenvironment. Finally, primary tumors can be effectively suppressed while causing minimal systemic toxicity. The distant tumors that are established after treatment can also be inhibited completely via activation of antitumor immunity in mice. Thus, the tumor-dilatable polymersome nanofactories with long-term intratumoral retention offer a promising paradigm for enhanced enzyme prodrug chemo-immunotherapy.
中文翻译:

用于增强酶前药化学免疫疗法的肿瘤扩张聚合物小体纳米工厂
癌症联合化学免疫疗法因其显着的协同抗肿瘤作用而备受关注。与化学疗法适当结合后,免疫疗法的反应率和治疗效果可显着提高。然而,化学免疫疗法经常受到严重的免疫相关不良事件和全身副作用的限制。在本报告中,基于具有优化膜交联密度的酶负载肿瘤可扩张聚合物囊泡,证明了有效的纳米工厂导向的酶前药化学免疫疗法。静脉注射纳米工厂后,它们可以被动地聚集在肿瘤部位。在肿瘤酸度的触发下,肿瘤 pH 响应性纳米工厂可以从~100 nm 膨胀到~200 nm,导致在肿瘤组织内的停留时间延长长达一周。同时,纳米工厂的膜渗透性显着提高,允许亲水性小分子穿过膜,同时将酶保持在内腔中。随后,在 6 天内施用 3 次化学免疫疗法的无毒前药混合物,它们是由纳米工厂在肿瘤部位选择性地原位激活。活化的化疗药物杀死癌细胞并产生肿瘤相关抗原以促进树突细胞的成熟。活化的吲哚胺 2, 3-双加氧酶 1 抑制剂逆转免疫抑制性肿瘤微环境。最后,可以有效抑制原发性肿瘤,同时引起最小的全身毒性。治疗后形成的远处肿瘤也可以通过激活小鼠抗肿瘤免疫来完全抑制。因此,具有长期肿瘤内保留的肿瘤可扩张聚合物体纳米工厂为增强的酶前药化学免疫疗法提供了有希望的范例。









































 京公网安备 11010802027423号
京公网安备 11010802027423号