当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of an E. coli strain for cell-free ADC manufacturing
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-10-16 , DOI: 10.1002/bit.27961 Dan Groff 1 , Nina A Carlos 1 , Rishard Chen 1 , Jeffrey A Hanson 1 , Shengwen Liang 2 , Stephanie Armstrong 1 , Xiaofan Li 1 , Sihong Zhou 1 , Alex Steiner 1 , Trevor J Hallam 1 , Gang Yin 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-10-16 , DOI: 10.1002/bit.27961 Dan Groff 1 , Nina A Carlos 1 , Rishard Chen 1 , Jeffrey A Hanson 1 , Shengwen Liang 2 , Stephanie Armstrong 1 , Xiaofan Li 1 , Sihong Zhou 1 , Alex Steiner 1 , Trevor J Hallam 1 , Gang Yin 1
Affiliation
|
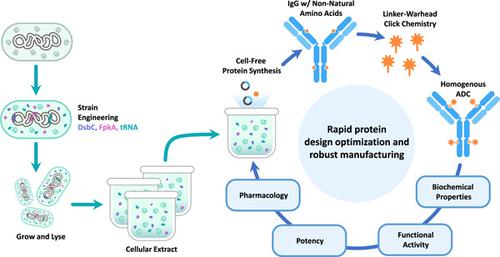
Recent advances in cell-free protein synthesis have enabled the folding and assembly of full-length antibodies at high titers with extracts from prokaryotic cells. Coupled with the facile engineering of the Escherichia coli translation machinery, E. coli based in vitro protein synthesis reactions have emerged as a leading source of IgG molecules with nonnatural amino acids incorporated at specific locations for producing homogeneous antibody–drug conjugates (ADCs). While this has been demonstrated with extract produced in batch fermentation mode, continuous extract fermentation would facilitate supplying material for large-scale manufacturing of protein therapeutics. To accomplish this, the IgG-folding chaperones DsbC and FkpA, and orthogonal tRNA for nonnatural amino acid production were integrated onto the chromosome with high strength constitutive promoters. This enabled co-expression of all three factors at a consistently high level in the extract strain for the duration of a 5-day continuous fermentation. Cell-free protein synthesis reactions with extract produced from cells grown continuously yielded titers of IgG containing nonnatural amino acids above those from extract produced in batch fermentations. In addition, the quality of the synthesized IgGs and the potency of ADC produced with continuously fermented extract were indistinguishable from those produced with the batch extract. These experiments demonstrate that continuous fermentation of E. coli to produce extract for cell-free protein synthesis is feasible and helps unlock the potential for cell-free protein synthesis as a platform for biopharmaceutical production.
中文翻译:

开发用于无细胞 ADC 制造的大肠杆菌菌株
无细胞蛋白质合成的最新进展使得能够用原核细胞提取物以高滴度折叠和组装全长抗体。再加上大肠杆菌翻译机器的简易工程,大肠杆菌基于体外蛋白质合成反应已成为 IgG 分子的主要来源,该分子在特定位置掺入非天然氨基酸,用于生产均质抗体-药物偶联物 (ADC)。虽然这已通过以分批发酵模式生产的提取物得到证明,但连续提取物发酵将有助于为蛋白质治疗剂的大规模生产提供材料。为此,将 IgG 折叠伴侣 DsbC 和 FkpA 以及用于非天然氨基酸生产的正交 tRNA 整合到具有高强度组成型启动子的染色体上。这使得在 5 天的连续发酵期间,所有三种因子在提取菌株中始终保持高水平的共表达。与从连续生长的细胞中产生的提取物进行无细胞蛋白质合成反应产生的含有非天然氨基酸的 IgG 滴度高于从分批发酵中产生的提取物的滴度。此外,用连续发酵提取物生产的合成 IgG 的质量和 ADC 的效力与用分批提取物生产的没有区别。这些实验表明,连续发酵大肠杆菌生产用于无细胞蛋白质合成的提取物是可行的,并有助于释放无细胞蛋白质合成作为生物制药生产平台的潜力。
更新日期:2021-12-04
中文翻译:

开发用于无细胞 ADC 制造的大肠杆菌菌株
无细胞蛋白质合成的最新进展使得能够用原核细胞提取物以高滴度折叠和组装全长抗体。再加上大肠杆菌翻译机器的简易工程,大肠杆菌基于体外蛋白质合成反应已成为 IgG 分子的主要来源,该分子在特定位置掺入非天然氨基酸,用于生产均质抗体-药物偶联物 (ADC)。虽然这已通过以分批发酵模式生产的提取物得到证明,但连续提取物发酵将有助于为蛋白质治疗剂的大规模生产提供材料。为此,将 IgG 折叠伴侣 DsbC 和 FkpA 以及用于非天然氨基酸生产的正交 tRNA 整合到具有高强度组成型启动子的染色体上。这使得在 5 天的连续发酵期间,所有三种因子在提取菌株中始终保持高水平的共表达。与从连续生长的细胞中产生的提取物进行无细胞蛋白质合成反应产生的含有非天然氨基酸的 IgG 滴度高于从分批发酵中产生的提取物的滴度。此外,用连续发酵提取物生产的合成 IgG 的质量和 ADC 的效力与用分批提取物生产的没有区别。这些实验表明,连续发酵大肠杆菌生产用于无细胞蛋白质合成的提取物是可行的,并有助于释放无细胞蛋白质合成作为生物制药生产平台的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号