当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Bioinorganic Chemistry of Mammalian Metallothioneins
Chemical Reviews ( IF 51.4 ) Pub Date : 2021-10-15 , DOI: 10.1021/acs.chemrev.1c00371 Artur Krężel 1 , Wolfgang Maret 2
Chemical Reviews ( IF 51.4 ) Pub Date : 2021-10-15 , DOI: 10.1021/acs.chemrev.1c00371 Artur Krężel 1 , Wolfgang Maret 2
Affiliation
|
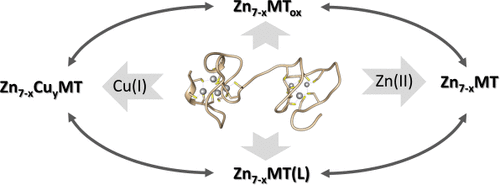
The functions, purposes, and roles of metallothioneins have been the subject of speculations since the discovery of the protein over 60 years ago. This article guides through the history of investigations and resolves multiple contentions by providing new interpretations of the structure-stability-function relationship. It challenges the dogma that the biologically relevant structure of the mammalian proteins is only the one determined by X-ray diffraction and NMR spectroscopy. The terms metallothionein and thionein are ambiguous and insufficient to understand biological function. The proteins need to be seen in their biological context, which limits and defines the chemistry possible. They exist in multiple forms with different degrees of metalation and types of metal ions. The homoleptic thiolate coordination of mammalian metallothioneins is important for their molecular mechanism. It endows the proteins with redox activity and a specific pH dependence of their metal affinities. The proteins, therefore, also exist in different redox states of the sulfur donor ligands. Their coordination dynamics allows a vast conformational landscape for interactions with other proteins and ligands. Many fundamental signal transduction pathways regulate the expression of the dozen of human metallothionein genes. Recent advances in understanding the control of cellular zinc and copper homeostasis are the foundation for suggesting that mammalian metallothioneins provide a highly dynamic, regulated, and uniquely biological metal buffer to control the availability, fluctuations, and signaling transients of the most competitive Zn(II) and Cu(I) ions in cellular space and time.
中文翻译:

哺乳动物金属硫蛋白的生物无机化学
自从 60 多年前发现这种蛋白质以来,金属硫蛋白的功能、用途和作用一直是人们猜测的主题。本文通过对结构-稳定性-功能关系的新解释来引导研究历史并解决多个争论。它挑战了哺乳动物蛋白质的生物学相关结构仅是由 X 射线衍射和 NMR 光谱确定的教条。术语金属硫蛋白和硫蛋白含糊不清,不足以理解生物学功能。蛋白质需要在其生物学背景下进行观察,这限制并定义了可能的化学反应。它们以多种形式存在,具有不同的金属化程度和金属离子类型。哺乳动物金属硫蛋白的同配硫醇配位对其分子机制很重要。它赋予蛋白质氧化还原活性和其金属亲和力的特定 pH 依赖性。因此,蛋白质也以硫供体配体的不同氧化还原状态存在。它们的配位动力学为与其他蛋白质和配体的相互作用提供了广阔的构象景观。许多基本的信号转导途径调节十几个人类金属硫蛋白基因的表达。了解细胞锌和铜稳态控制的最新进展是表明哺乳动物金属硫蛋白提供高度动态、可调节和独特的生物金属缓冲液以控制可用性、波动、
更新日期:2021-12-08
中文翻译:

哺乳动物金属硫蛋白的生物无机化学
自从 60 多年前发现这种蛋白质以来,金属硫蛋白的功能、用途和作用一直是人们猜测的主题。本文通过对结构-稳定性-功能关系的新解释来引导研究历史并解决多个争论。它挑战了哺乳动物蛋白质的生物学相关结构仅是由 X 射线衍射和 NMR 光谱确定的教条。术语金属硫蛋白和硫蛋白含糊不清,不足以理解生物学功能。蛋白质需要在其生物学背景下进行观察,这限制并定义了可能的化学反应。它们以多种形式存在,具有不同的金属化程度和金属离子类型。哺乳动物金属硫蛋白的同配硫醇配位对其分子机制很重要。它赋予蛋白质氧化还原活性和其金属亲和力的特定 pH 依赖性。因此,蛋白质也以硫供体配体的不同氧化还原状态存在。它们的配位动力学为与其他蛋白质和配体的相互作用提供了广阔的构象景观。许多基本的信号转导途径调节十几个人类金属硫蛋白基因的表达。了解细胞锌和铜稳态控制的最新进展是表明哺乳动物金属硫蛋白提供高度动态、可调节和独特的生物金属缓冲液以控制可用性、波动、









































 京公网安备 11010802027423号
京公网安备 11010802027423号