Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Subcellular Dynamic Immunopatterning of Cytosolic Protein Complexes on Microstructured Polymer Substrates
ACS Sensors ( IF 8.2 ) Pub Date : 2021-10-15 , DOI: 10.1021/acssensors.1c01574 Roland Hager 1 , Ulrike Müller 1 , Nicole Ollinger 2 , Julian Weghuber 1, 2 , Peter Lanzerstorfer 1
ACS Sensors ( IF 8.2 ) Pub Date : 2021-10-15 , DOI: 10.1021/acssensors.1c01574 Roland Hager 1 , Ulrike Müller 1 , Nicole Ollinger 2 , Julian Weghuber 1, 2 , Peter Lanzerstorfer 1
Affiliation
|
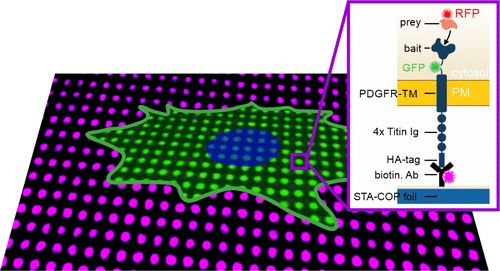
Analysis of protein–protein interactions in living cells by protein micropatterning is currently limited to the spatial arrangement of transmembrane proteins and their corresponding downstream molecules. Here, we present a robust and straightforward method for dynamic immunopatterning of cytosolic protein complexes by use of an artificial transmembrane bait construct in combination with microstructured antibody arrays on cyclic olefin polymer substrates. As a proof, the method was used to characterize Grb2-mediated signaling pathways downstream of the epidermal growth factor receptor (EGFR). Ternary protein complexes (Shc1:Grb2:SOS1 and Grb2:Gab1:PI3K) were identified, and we found that EGFR downstream signaling is based on constitutively bound (Grb2:SOS1 and Grb2:Gab1) as well as on agonist-dependent protein associations with transient interaction properties (Grb2:Shc1 and Grb2:PI3K). Spatiotemporal analysis further revealed significant differences in stability and exchange kinetics of protein interactions. Furthermore, we could show that this approach is well suited to study the efficacy and specificity of SH2 and SH3 protein domain inhibitors in a live cell context. Altogether, this method represents a significant enhancement of quantitative subcellular micropatterning approaches as an alternative to standard biochemical analyses.
中文翻译:

细胞质蛋白复合物在微结构聚合物底物上的亚细胞动态免疫模式化
通过蛋白质微模式分析活细胞中的蛋白质-蛋白质相互作用目前仅限于跨膜蛋白质及其相应下游分子的空间排列。在这里,我们提出了一种稳健而直接的方法,通过使用人工跨膜诱饵构建体结合环烯烃聚合物基材上的微结构抗体阵列,对胞质蛋白复合物进行动态免疫模式化。作为证明,该方法用于表征表皮生长因子受体 (EGFR) 下游的 Grb2 介导的信号通路。鉴定了三元蛋白复合物(Shc1:Grb2:SOS1 和 Grb2:Gab1:PI3K),我们发现 EGFR 下游信号是基于组成性结合的(Grb2:SOS1 和 Grb2:Gab1) 以及具有瞬时相互作用特性的激动剂依赖性蛋白质关联 (Grb2:Shc1 和 Grb2:PI3K)。时空分析进一步揭示了蛋白质相互作用的稳定性和交换动力学的显着差异。此外,我们可以证明这种方法非常适合在活细胞环境中研究 SH2 和 SH3 蛋白域抑制剂的功效和特异性。总而言之,这种方法代表了定量亚细胞微模式化方法的显着增强,作为标准生化分析的替代方法。我们可以证明这种方法非常适合在活细胞环境中研究 SH2 和 SH3 蛋白结构域抑制剂的功效和特异性。总而言之,这种方法代表了定量亚细胞微模式化方法的显着增强,作为标准生化分析的替代方法。我们可以证明这种方法非常适合在活细胞环境中研究 SH2 和 SH3 蛋白结构域抑制剂的功效和特异性。总而言之,这种方法代表了定量亚细胞微模式化方法的显着增强,作为标准生化分析的替代方法。
更新日期:2021-11-26
中文翻译:

细胞质蛋白复合物在微结构聚合物底物上的亚细胞动态免疫模式化
通过蛋白质微模式分析活细胞中的蛋白质-蛋白质相互作用目前仅限于跨膜蛋白质及其相应下游分子的空间排列。在这里,我们提出了一种稳健而直接的方法,通过使用人工跨膜诱饵构建体结合环烯烃聚合物基材上的微结构抗体阵列,对胞质蛋白复合物进行动态免疫模式化。作为证明,该方法用于表征表皮生长因子受体 (EGFR) 下游的 Grb2 介导的信号通路。鉴定了三元蛋白复合物(Shc1:Grb2:SOS1 和 Grb2:Gab1:PI3K),我们发现 EGFR 下游信号是基于组成性结合的(Grb2:SOS1 和 Grb2:Gab1) 以及具有瞬时相互作用特性的激动剂依赖性蛋白质关联 (Grb2:Shc1 和 Grb2:PI3K)。时空分析进一步揭示了蛋白质相互作用的稳定性和交换动力学的显着差异。此外,我们可以证明这种方法非常适合在活细胞环境中研究 SH2 和 SH3 蛋白域抑制剂的功效和特异性。总而言之,这种方法代表了定量亚细胞微模式化方法的显着增强,作为标准生化分析的替代方法。我们可以证明这种方法非常适合在活细胞环境中研究 SH2 和 SH3 蛋白结构域抑制剂的功效和特异性。总而言之,这种方法代表了定量亚细胞微模式化方法的显着增强,作为标准生化分析的替代方法。我们可以证明这种方法非常适合在活细胞环境中研究 SH2 和 SH3 蛋白结构域抑制剂的功效和特异性。总而言之,这种方法代表了定量亚细胞微模式化方法的显着增强,作为标准生化分析的替代方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号