当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insights into Dual-Metal-Site Catalysts for the Nonoxidative Coupling of Methane
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-14 , DOI: 10.1021/acscatal.1c02597 Zheng-Qing Huang 1 , You-Tao Chen 1, 2 , Chun-Ran Chang 1 , Jun Li 3, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-14 , DOI: 10.1021/acscatal.1c02597 Zheng-Qing Huang 1 , You-Tao Chen 1, 2 , Chun-Ran Chang 1 , Jun Li 3, 4
Affiliation
|
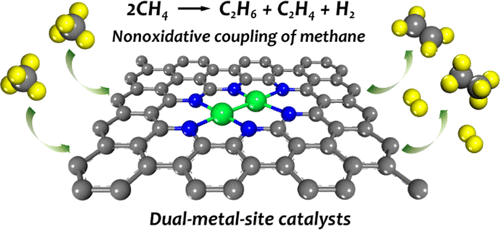
Direct conversion of methane to C2 hydrocarbons under nonoxidative conditions is an attractive technology but is challenging due to high reaction temperature, severe coke deposition, and low selectivity. Here, we report three dual-metal-site catalysts (DMSCs) based on nitrogen-doped graphene (FeCo–N–C, Fe2–N–C, and Co2–N–C) for nonoxidative coupling of methane to C2 hydrocarbons from a theoretical perspective. Our calculated results reveal that DMSCs present universally better performance in methane activation than single-metal-site catalysts (Fe–N–C and Co–N–C). Among the three DMSCs, Co2–N–C exhibits the best catalytic activity and superior selectivity to ethane in the whole reaction pathway. Our microkinetic modeling reveals that the Co2–N–C catalyst can convert methane to CH3, C2H6, and H2 at 1200 K. The electronic structure analysis and ab initio molecular dynamics simulations demonstrate that Co2–N–C possesses both intrinsic stability and high-temperature stability. Moreover, Co2–N–C also manifests better coke resistance compared with larger Co clusters, indicated by the difficult kinetics of methane deep dehydrogenation to naked carbon. This work provides a potential catalyst prototype for the selective conversion of methane to C2 hydrocarbons under nonoxidative conditions.
中文翻译:

用于甲烷非氧化偶联的双金属位点催化剂的理论见解
在非氧化条件下将甲烷直接转化为 C 2烃是一项有吸引力的技术,但由于反应温度高、焦炭沉积严重和选择性低而具有挑战性。在这里,我们报告了三种基于氮掺杂石墨烯(FeCo–N–C、Fe 2 –N–C 和 Co 2 –N–C)的双金属位点催化剂(DMSCs),用于甲烷与 C 2 的非氧化偶联从理论角度看碳氢化合物。我们的计算结果表明,与单金属位点催化剂(Fe-N-C 和 Co-N-C)相比,DMSCs 在甲烷活化方面表现出普遍更好的性能。在三个 DMSC 中,Co 2-N-C在整个反应途径中表现出最好的催化活性和对乙烷的优异选择性。我们的微动力学模型表明,Co 2 -N-C 催化剂可以在 1200 K 下将甲烷转化为 CH 3、C 2 H 6和 H 2。电子结构分析和从头算分子动力学模拟表明 Co 2 -N-C兼具内在稳定性和高温稳定性。此外,Co 2与较大的 Co 簇相比,–N–C 还表现出更好的抗焦炭性,这由甲烷深度脱氢成裸碳的困难动力学表明。这项工作为在非氧化条件下将甲烷选择性转化为 C 2烃提供了一种潜在的催化剂原型。
更新日期:2021-11-05
中文翻译:

用于甲烷非氧化偶联的双金属位点催化剂的理论见解
在非氧化条件下将甲烷直接转化为 C 2烃是一项有吸引力的技术,但由于反应温度高、焦炭沉积严重和选择性低而具有挑战性。在这里,我们报告了三种基于氮掺杂石墨烯(FeCo–N–C、Fe 2 –N–C 和 Co 2 –N–C)的双金属位点催化剂(DMSCs),用于甲烷与 C 2 的非氧化偶联从理论角度看碳氢化合物。我们的计算结果表明,与单金属位点催化剂(Fe-N-C 和 Co-N-C)相比,DMSCs 在甲烷活化方面表现出普遍更好的性能。在三个 DMSC 中,Co 2-N-C在整个反应途径中表现出最好的催化活性和对乙烷的优异选择性。我们的微动力学模型表明,Co 2 -N-C 催化剂可以在 1200 K 下将甲烷转化为 CH 3、C 2 H 6和 H 2。电子结构分析和从头算分子动力学模拟表明 Co 2 -N-C兼具内在稳定性和高温稳定性。此外,Co 2与较大的 Co 簇相比,–N–C 还表现出更好的抗焦炭性,这由甲烷深度脱氢成裸碳的困难动力学表明。这项工作为在非氧化条件下将甲烷选择性转化为 C 2烃提供了一种潜在的催化剂原型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号