当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Demonstration of Controlled Hydrogen Release Using Rh@GQDs during Hydrolysis of NH3BH3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-10-15 , DOI: 10.1021/acsami.1c15660 Weifeng Chen 1 , Guo Lv 1 , Jinrun Fu 1 , Haiyan Ren 1 , Jialu Shen 1 , Jie Cao 1 , Xiang Liu 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-10-15 , DOI: 10.1021/acsami.1c15660 Weifeng Chen 1 , Guo Lv 1 , Jinrun Fu 1 , Haiyan Ren 1 , Jialu Shen 1 , Jie Cao 1 , Xiang Liu 1
Affiliation
|
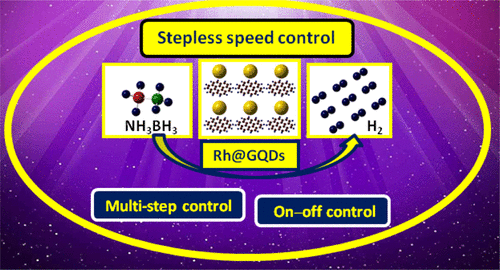
Achieving the controlled release of H2 through an effective approach still faces many challenges. Herein, high-quality graphene quantum dots (GQDs) are synthesized from a new precursor, 1,2,4-trihydroxy benzene, and a multifunctional platform of Rh@GQDs is further developed for the controlled H2 evolution upon the hydrolysis of NH3BH3 (AB). More importantly, the designing concepts of multistep and stepless speed controls have been introduced in the domains of both H2 evolution for the first time. Through a novel designing protocol, the rate of H2 evolution can be freely regulated and constantly varied on demand by means of chelation between Zn2+ and ethylene diamine tetraacetic acid (EDTA). The density functional theory calculation indicates that Zn2+ has the priority to be adsorbed onto Rh(100) due to its larger adsorption energy (107.98 kcal·mol–1) than that of AB (36.36 kcal·mol–1). A controlling mechanism is presented such that Zn2+ will cover the active sites of the nanocatalyst to prevent the H2 evolution, and EDTA can chelate Zn2+ to reactivate the nanocatalyst for the production of H2, greatly facilitating use of this strategy in other catalytic reactions. Moreover, it is demonstrated that the protocol is equally valid for diverse hydrogen storage materials. Therefore, this work not only establishes whole new concepts for the controlled production of H2 but also explains their mechanism, thus remarkably advancing the utilization of H2 energy and significantly enlightening the controlled process of catalysis.
中文翻译:

在 NH3BH3 的水解过程中使用 Rh@GQDs 控制氢释放的演示
通过有效的方法实现H 2 的控释仍面临许多挑战。在这里,高质量的石墨烯量子点 (GQDs) 由一种新的前驱体 1,2,4-三羟基苯合成,并进一步开发了一个多功能的 Rh@GQDs 平台,用于控制NH 3水解时的H 2析出BH 3 (AB)。更重要的是,首次在H 2演化领域引入了多级和无级调速的设计理念。通过一种新颖的设计方案,H 2的生成速率可以通过Zn 2+之间的螯合来自由调节并根据需要不断变化和乙二胺四乙酸 (EDTA)。密度泛函理论计算表明,Zn 2+由于其吸附能(107.98 kcal·mol –1)比AB(36.36 kcal·mol –1)大,因此优先被吸附在Rh(100 )上。提出了一种控制机制,即 Zn 2+将覆盖纳米催化剂的活性位点以防止 H 2析出,并且 EDTA 可以螯合 Zn 2+以重新激活纳米催化剂以产生 H 2,极大地促进了该策略在其他催化反应中的使用。此外,证明该协议对不同的储氢材料同样有效。因此,这项工作不仅为H 2的可控生产建立了全新的概念,而且还解释了其机理,从而显着促进了H 2能量的利用,对催化过程的控制具有重要意义。
更新日期:2021-10-27
中文翻译:

在 NH3BH3 的水解过程中使用 Rh@GQDs 控制氢释放的演示
通过有效的方法实现H 2 的控释仍面临许多挑战。在这里,高质量的石墨烯量子点 (GQDs) 由一种新的前驱体 1,2,4-三羟基苯合成,并进一步开发了一个多功能的 Rh@GQDs 平台,用于控制NH 3水解时的H 2析出BH 3 (AB)。更重要的是,首次在H 2演化领域引入了多级和无级调速的设计理念。通过一种新颖的设计方案,H 2的生成速率可以通过Zn 2+之间的螯合来自由调节并根据需要不断变化和乙二胺四乙酸 (EDTA)。密度泛函理论计算表明,Zn 2+由于其吸附能(107.98 kcal·mol –1)比AB(36.36 kcal·mol –1)大,因此优先被吸附在Rh(100 )上。提出了一种控制机制,即 Zn 2+将覆盖纳米催化剂的活性位点以防止 H 2析出,并且 EDTA 可以螯合 Zn 2+以重新激活纳米催化剂以产生 H 2,极大地促进了该策略在其他催化反应中的使用。此外,证明该协议对不同的储氢材料同样有效。因此,这项工作不仅为H 2的可控生产建立了全新的概念,而且还解释了其机理,从而显着促进了H 2能量的利用,对催化过程的控制具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号