The Journal of Supercritical Fluids ( IF 3.4 ) Pub Date : 2021-10-14 , DOI: 10.1016/j.supflu.2021.105447 Gabriel V.S. Seufitelli 1 , Fernando L.P. Resende 2 , Rick Gustafson 1
|
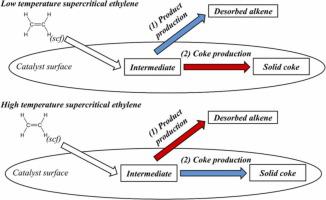
We investigated the effect of coke solubility in supercritical ethylene on the ethylene oligomerization over nickel-based heterogeneous catalysts. The approach uses n-decane as a model compound for coke to simulate coke formed during the catalytic process. We report the solubility of n-decane in ethylene at 30, 50, and 75 oC and pressures ranging from 1 to 68 bar; conditions previously screened by our research group for ethylene oligomerization. We reproduced previous literature data on the solubility of n-decane in nitrogen to validate the flow system designed in the present work. The present study is the first to report the solubility of n-decane in ethylene under a broad range of conditions, including subcritical and supercritical conditions. We found that the ethylene – n-decane system deviates from ideality very quickly with increasing pressure. Beyond the ethylene critical point (P = 50.3 bar and T = 9.4 oC) the solubility (expressed in terms of mole fraction) of n-decane in ethylene at 30 oC reaches a maximum value of 3.0%; which is close to the value observed at 50 and 75 oC, under the same pressure. We used the solubility data to estimate the rate of coke dissolution and compared these values with kinetic data previously reported by our group under supercritical conditions. Analysis of coke dissolution rates in supercritical ethylene under different temperatures indicates that the transport of products from the catalyst surface to the bulk of supercritical ethylene controls the catalytic process at supercritical conditions. The approach used in the present work is novel because it accounts for the contribution of coke dissolution rates on the ethylene oligomerization over nickel-based solid catalysts..
中文翻译:

正癸烷在乙烯中的溶解度及其对多相催化剂上超临界乙烯齐聚反应的影响
我们研究了焦炭在超临界乙烯中的溶解度对镍基多相催化剂上乙烯低聚反应的影响。该方法使用正癸烷作为焦炭的模型化合物来模拟在催化过程中形成的焦炭。我们报告了正癸烷在 30、50 和 75 o C 和 1 至 68 bar 压力下在乙烯中 的溶解度;我们的研究小组先前筛选的乙烯低聚反应条件。我们复制了之前关于正癸烷在氮气中的溶解度的文献数据,以验证当前工作中设计的流动系统。本研究首次报道了n-在广泛的条件下,包括亚临界和超临界条件下,乙烯中的癸烷。我们发现乙烯-正癸烷系统随着压力的增加很快偏离理想状态。超过乙烯临界点(P = 50.3 bar 和 T = 9.4 o C),正癸烷在 30 o C 下在乙烯中 的溶解度(以摩尔分数表示)达到最大值 3.0%;这接近在 50 和 75 o观察到的值 C、在同样的压力下。我们使用溶解度数据来估计焦炭溶解速率,并将这些值与我们小组先前在超临界条件下报告的动力学数据进行比较。不同温度下超临界乙烯中焦炭溶解速率的分析表明,产物从催化剂表面到超临界乙烯本体的传输控制了超临界条件下的催化过程。本工作中使用的方法是新颖的,因为它解释了焦炭溶解速率对镍基固体催化剂上乙烯低聚的贡献。










































 京公网安备 11010802027423号
京公网安备 11010802027423号