Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2021-10-14 , DOI: 10.1016/j.cej.2021.132980 Hao Li 1 , Xin Xu 1 , Xue Liu 1 , Zhen Yao 1 , Kun Cao 1, 2
|
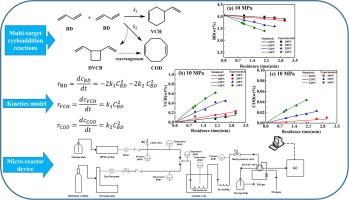
The kinetics of 1,3-butadiene dimerization based on the cycloaddition reactions including Diels-Alder reaction under high temperature and high pressure were investigated in a microchannel reactor. Our findings suggest that the butadiene dimerization produced 4-vinyl-cyclohexene (VCH) and 1,5-cyclooctadiene (COD). The results indicate that COD can only be formed under high temperature conditions. The apparent kinetic parameters of butadiene dimerization under high pressure were obtained by fitting experimental data. As the reaction pressure increased, the reaction rate in the solution was found to increase slightly. Compared with the gas phase dimerization of butadiene in the literature, it can be concluded that the reaction rate constants in the liquid phase are greater than that in the gas phase. To our best knowledge, this is the first work reporting the kinetic parameters of butadiene dimerization with the radical polymerization excluded under high pressure liquid phase condition.
中文翻译:

高压高温微反应器环加成反应1,3-丁二烯二聚反应动力学
在微通道反应器中研究了基于Diels-Alder反应等环加成反应的1,3-丁二烯二聚反应动力学。我们的研究结果表明,丁二烯二聚反应产生了 4-乙烯基-环己烯 (VCH) 和 1,5- 环辛二烯 (COD)。结果表明,COD 只能在高温条件下形成。通过拟合实验数据,得到了高压下丁二烯二聚反应的表观动力学参数。随着反应压力的增加,发现溶液中的反应速率略有增加。与文献中丁二烯的气相二聚相比,可以得出结论,液相中的反应速率常数大于气相中的反应速率常数。据我们所知,



























 京公网安备 11010802027423号
京公网安备 11010802027423号