Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-10-13 , DOI: 10.1016/j.jhazmat.2021.127491 Jianxin Pan 1 , Liangliang Liu 1 , Hanping Pan 2 , Lihui Yang 1 , Meirong Su 1 , Chaohai Wei 3
|
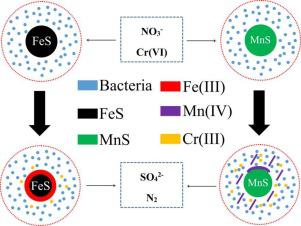
Metal sulfide-based biological process is considered as a promising biotechnology for next-generation wastewater treatment. However, it is not clear if simultaneous bio-reduction of nitrate and chromate was achievable in this process. This study aimed to evaluate the feasibility of metal sulfides (FeS and MnS) on simultaneous denitrification and chromate reduction in autotrophic denitrifying column bioreactors. Results showed that simultaneous reduction of nitrate and chromate was achieved using metal sulfides (FeS and MnS) as electron donors, in which sulfate was the sole soluble end-product. Apart from the sulfur element in the metal sulfides, Fe(II) and Mn(II) were also involved in nitrate and chromate reduction as indicative by the formation of their oxidative states compounds. In microbial communities, SHD-231 and Thiobacillus were the most predominant bacteria, which might have played important roles in simultaneous denitrification and chromate reduction. Compared to FeS, MnS showed a higher performance on nitrate and chromate removal, which could also reduce the toxic inhibition of chromate on nitrate reduction. According to results of XRD and XPS, as well as a lower sulfate production in the FeS system, FeS might have been covered easily to hydroxides due to its bio-oxidation, which limited mass transfer efficiency and bio-availability of FeS. The findings in this study offered insights in the development of promising approaches for the treatment of toxic and hazardous compounds using metal sulfide.
中文翻译:

金属硫化物(FeS和MnS)同时反硝化和铬酸盐还原的可行性研究
基于金属硫化物的生物工艺被认为是用于下一代废水处理的有前途的生物技术。然而,尚不清楚在这个过程中是否可以同时实现硝酸盐和铬酸盐的生物还原。本研究旨在评估金属硫化物(FeS 和 MnS)在自养反硝化柱生物反应器中同时反硝化和铬酸盐还原的可行性。结果表明,使用金属硫化物(FeS 和 MnS)作为电子供体可以同时还原硝酸盐和铬酸盐,其中硫酸盐是唯一可溶的最终产物。除了金属硫化物中的硫元素外,Fe(II) 和 Mn(II) 也参与了硝酸盐和铬酸盐的还原,这表明它们形成了氧化态化合物。在微生物群落中,SHD-231和硫杆菌是最主要的细菌,它们可能在同时反硝化和铬酸盐还原中起重要作用。与FeS相比,MnS对硝酸盐和铬酸盐的去除表现出更高的性能,这也可以降低铬酸盐对硝酸盐还原的毒性抑制作用。根据 XRD 和 XPS 的结果,以及 FeS 体系中较低的硫酸盐产量,FeS 可能由于其生物氧化作用而容易被氢氧化物覆盖,这限制了 FeS 的传质效率和生物利用度。本研究中的发现为开发使用金属硫化物处理有毒和有害化合物的有前景的方法提供了见解。MnS对硝酸盐和铬酸盐的去除表现出更高的性能,这也可以降低铬酸盐对硝酸盐还原的毒性抑制作用。根据 XRD 和 XPS 的结果,以及 FeS 体系中较低的硫酸盐产量,FeS 可能由于其生物氧化作用而容易被氢氧化物覆盖,这限制了 FeS 的传质效率和生物利用度。本研究中的发现为开发使用金属硫化物处理有毒和有害化合物的有前景的方法提供了见解。MnS对硝酸盐和铬酸盐的去除表现出更高的性能,这也可以降低铬酸盐对硝酸盐还原的毒性抑制作用。根据 XRD 和 XPS 的结果,以及 FeS 体系中较低的硫酸盐产量,FeS 可能由于其生物氧化作用而容易被氢氧化物覆盖,这限制了 FeS 的传质效率和生物利用度。本研究中的发现为开发使用金属硫化物处理有毒和有害化合物的有前景的方法提供了见解。这限制了 FeS 的传质效率和生物利用度。本研究中的发现为开发使用金属硫化物处理有毒和有害化合物的有前景的方法提供了见解。这限制了 FeS 的传质效率和生物利用度。本研究中的发现为开发使用金属硫化物处理有毒和有害化合物的有前景的方法提供了见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号