Journal of Controlled Release ( IF 10.5 ) Pub Date : 2021-10-13 , DOI: 10.1016/j.jconrel.2021.10.004 Runyu Zhang 1 , Hailiang Deng 1 , Yuxing Lin 1 , Xing Wang 1 , Bing He 2 , Wenbing Dai 1 , Hua Zhang 1 , Ying Zheng 3 , Qiang Zhang 2 , Xueqing Wang 1
|
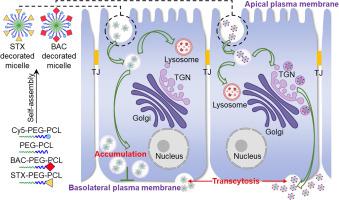
The intestinal barrier has always been the rate-limiting step in the oral administration process. To overcome the intestinal barrier, researchers have widely adopted nanocarriers, especially active-targeting nanocarriers strategies. However, most of these strategies focus on the ligand decoration of nanocarriers targeting specific receptors, so their applications are confined to specific receptors or specific cell types. In this study, we tried to investigate more common strategies in the field of transmembrane transport enhancement. Trans-Golgi network (TGN) is the sorting center of biosynthetic route which could achieve polarized localization of proteins in polarized epithelial cells, and the basolateral plasma membrane is where all transcytotic cargos have to pass through. Thus, it is expected that guiding nanocarriers to TGN or basolateral plasma membrane may improve the transcytosis. Hence, we choose sorting signal peptide to modify micelles to guide micelles to TGN (named as BAC decorated micelles, BAC-M) or to basolateral plasma membrane (named as STX decorated micelles, STX-M). By incorporating coumarin-6 (C6) or Cy5-PEG-PCL in the micelles to indicate the behavior of micelles, the effects of these two strategies on the transcytosis were investigated. To our surprise, BAC-M and STX-M behaved quite differently when crossing biological barriers. BAC-M showed significant superiority in colocalization with TGN, transmembrane transport and even in vivo absorption, while STX-M had no significant difference from blank micelles. Further investigation revealed that the strategy of directly guiding nanocarriers to the basolateral plasma membrane (STX-M) only caused the stack of vesicles near the basolateral plasma membrane. So, we concluded that guiding nanocarriers to TGN which related to secretion may contribute to the transmembrane transport. This common strategy based on the physiological function of TGN in polarized epithelial cells will have broad application prospects in overcoming biological barrier.
中文翻译:

基于分选信号改善极化上皮细胞跨膜转运的常见策略:将纳米载体引导至 TGN 而不是直接引导至基底外侧质膜
肠道屏障一直是口服给药过程中的限速步骤。为了克服肠道屏障,研究人员广泛采用纳米载体,尤其是主动靶向纳米载体策略。然而,这些策略大多集中在靶向特定受体的纳米载体的配体修饰上,因此它们的应用仅限于特定受体或特定细胞类型。在这项研究中,我们试图研究跨膜转运增强领域中更常见的策略。反式-高尔基网络(TGN)是生物合成途径的分选中心,可以实现极化上皮细胞中蛋白质的极化定位,基底外侧质膜是所有转运货物必须通过的地方。因此,预计将纳米载体引导至 TGN 或基底外侧质膜可以改善胞吞作用。因此,我们选择分选信号肽来修饰胶束以将胶束引导至 TGN(称为 BAC 修饰胶束,BAC-M)或基底外侧质膜(称为 STX 修饰胶束,STX-M)。通过在胶束中加入香豆素-6 (C6) 或 Cy5-PEG-PCL 来指示胶束的行为,研究了这两种策略对转胞吞作用的影响。令我们惊讶的是,BAC-M 和 STX-M 在跨越生物屏障时的表现截然不同。体内吸收,而STX-M与空白胶束无显着差异。进一步的研究表明,直接将纳米载体引导至基底外侧质膜 (STX-M) 的策略仅导致基底外侧质膜附近的囊泡堆叠。因此,我们得出结论,将纳米载体引导至与分泌相关的 TGN 可能有助于跨膜转运。这种基于极化上皮细胞中 TGN 生理功能的通用策略将在克服生物屏障方面具有广阔的应用前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号