当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Energetics of GTP- and GDP-Tubulin Isodesmic Self-Association
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-10-13 , DOI: 10.1021/acschembio.1c00369 Asaf Shemesh 1, 2 , Avi Ginsburg 1 , Raviv Dharan 1 , Yael Levi-Kalisman 2, 3 , Israel Ringel 4 , Uri Raviv 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-10-13 , DOI: 10.1021/acschembio.1c00369 Asaf Shemesh 1, 2 , Avi Ginsburg 1 , Raviv Dharan 1 , Yael Levi-Kalisman 2, 3 , Israel Ringel 4 , Uri Raviv 1, 2
Affiliation
|
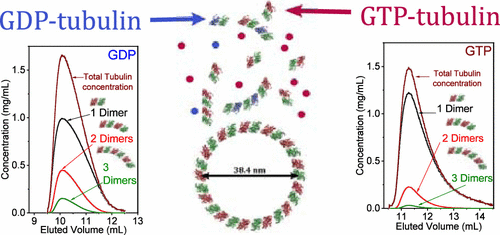
Tubulin self-association is a critical process in microtubule dynamics. The early intermediate structures, energetics, and their regulation by fluxes of chemical energy, associated with guanosine triphosphate (GTP) hydrolysis, are poorly understood. We reconstituted an in vitro minimal model system, mimicking the key elements of the nontemplated tubulin assembly. To resolve the distribution of GTP- and guanosine diphosphate (GDP)-tubulin structures, at low temperatures (∼10 °C) and below the critical concentration for the microtubule assembly, we analyzed in-line size-exclusion chromatography–small-angle X-ray scattering (SEC-SAXS) chromatograms of GTP- and GDP-tubulin solutions. Both solutions rapidly attained steady state. The SEC-SAXS data were consistent with an isodesmic thermodynamic model of longitudinal tubulin self-association into 1D oligomers, terminated by the formation of tubulin single rings. The analysis showed that free dimers coexisted with tetramers and hexamers. Tubulin monomers and lateral association between dimers were not detected. The dimer–dimer longitudinal self-association standard Helmholtz free energies were −14.2 ± 0.4 kBT (−8.0 ± 0.2 kcal mol–1) and −13.1 ± 0.5 kBT (−7.4 ± 0.3 kcal mol–1) for GDP- and GTP-tubulin, respectively. We then determined the mass fractions of dimers, tetramers, and hexamers as a function of the total tubulin concentration. A small fraction of stable tubulin single rings, with a radius of 19.2 ± 0.2 nm, was detected in the GDP-tubulin solution. In the GTP-tubulin solution, this fraction was significantly lower. Cryo-TEM images and SEC-multiangle light-scattering analysis corroborated these findings. Our analyses provide an accurate structure–stability description of cold tubulin solutions.
中文翻译:

GTP-和 GDP-微管蛋白等位自缔合的结构和能量学
微管蛋白自缔合是微管动力学中的一个关键过程。与三磷酸鸟苷 (GTP) 水解相关的早期中间结构、能量学及其对化学能通量的调节知之甚少。我们在体外重组了最小模型系统,模仿非模板微管蛋白组装的关键元素。为了解决 GTP-和鸟苷二磷酸 (GDP)-微管蛋白结构的分布,在低温 (~10 °C) 和低于微管组装的临界浓度下,我们分析了在线尺寸排阻色谱-小角度 X - GTP-和 GDP-微管蛋白溶液的射线散射 (SEC-SAXS) 色谱图。两种解决方案都迅速达到稳定状态。SEC-SAXS 数据与纵向微管蛋白自结合成 1D 低聚物的等速热力学模型一致,通过微管蛋白单环的形成终止。分析表明游离二聚体与四聚体和六聚体共存。未检测到微管蛋白单体和二聚体之间的横向结合。k B T (-8.0 ± 0.2 kcal mol –1 ) 和 -13.1 ± 0.5 k B T (-7.4 ± 0.3 kcal mol –1 ) 分别用于 GDP- 和 GTP-微管蛋白。然后,我们确定了二聚体、四聚体和六聚体的质量分数作为总微管蛋白浓度的函数。在 GDP-微管蛋白溶液中检测到一小部分稳定的微管蛋白单环,半径为 19.2 ± 0.2 nm。在 GTP-微管蛋白溶液中,这个分数显着降低。冷冻 TEM 图像和 SEC 多角度光散射分析证实了这些发现。我们的分析提供了冷微管蛋白溶液的准确结构稳定性描述。
更新日期:2021-11-19
中文翻译:

GTP-和 GDP-微管蛋白等位自缔合的结构和能量学
微管蛋白自缔合是微管动力学中的一个关键过程。与三磷酸鸟苷 (GTP) 水解相关的早期中间结构、能量学及其对化学能通量的调节知之甚少。我们在体外重组了最小模型系统,模仿非模板微管蛋白组装的关键元素。为了解决 GTP-和鸟苷二磷酸 (GDP)-微管蛋白结构的分布,在低温 (~10 °C) 和低于微管组装的临界浓度下,我们分析了在线尺寸排阻色谱-小角度 X - GTP-和 GDP-微管蛋白溶液的射线散射 (SEC-SAXS) 色谱图。两种解决方案都迅速达到稳定状态。SEC-SAXS 数据与纵向微管蛋白自结合成 1D 低聚物的等速热力学模型一致,通过微管蛋白单环的形成终止。分析表明游离二聚体与四聚体和六聚体共存。未检测到微管蛋白单体和二聚体之间的横向结合。k B T (-8.0 ± 0.2 kcal mol –1 ) 和 -13.1 ± 0.5 k B T (-7.4 ± 0.3 kcal mol –1 ) 分别用于 GDP- 和 GTP-微管蛋白。然后,我们确定了二聚体、四聚体和六聚体的质量分数作为总微管蛋白浓度的函数。在 GDP-微管蛋白溶液中检测到一小部分稳定的微管蛋白单环,半径为 19.2 ± 0.2 nm。在 GTP-微管蛋白溶液中,这个分数显着降低。冷冻 TEM 图像和 SEC 多角度光散射分析证实了这些发现。我们的分析提供了冷微管蛋白溶液的准确结构稳定性描述。









































 京公网安备 11010802027423号
京公网安备 11010802027423号