当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methanol-Mediated Electrosynthesis of Ammonia
ACS Energy Letters ( IF 22.0 ) Pub Date : 2021-10-11 , DOI: 10.1021/acsenergylett.1c01893 Yongwen Ren 1 , Chang Yu 1 , Xiaotong Han 1 , Xinyi Tan 1 , Qianbing Wei 1 , Wenbin Li 1 , Yingnan Han 1 , Le Yang 1 , Jieshan Qiu 1
ACS Energy Letters ( IF 22.0 ) Pub Date : 2021-10-11 , DOI: 10.1021/acsenergylett.1c01893 Yongwen Ren 1 , Chang Yu 1 , Xiaotong Han 1 , Xinyi Tan 1 , Qianbing Wei 1 , Wenbin Li 1 , Yingnan Han 1 , Le Yang 1 , Jieshan Qiu 1
Affiliation
|
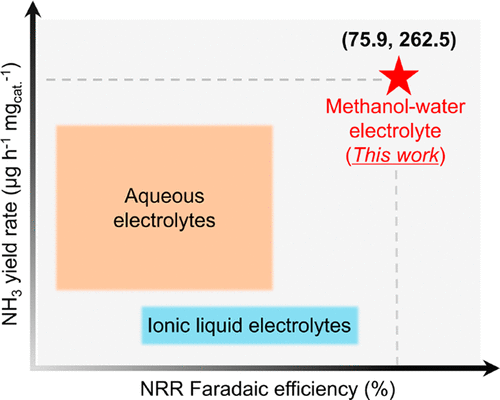
The development of electrochemical nitrogen reduction reaction (NRR) to ammonia currently faces the dilemma of low Faradaic efficiency (FE) due to the competing hydrogen evolution reaction (HER). The proton-donating ability of proton donor at the electrode–electrolyte interface plays a critical role in inhibiting HER and then boosting the selectivity of NRR. Depending on the intrinsic discrepancy of proton-donating ability between alcohol and water, herein, we demonstrate an innovatively well-controlled alcohol–water electrolyte system to modulate local proton concentration and the microenvironment at the electrode–electrolyte interface, wherein the availability and dissociation process of water can be substantially restricted, accompanied by an expanded electrochemical window and inhibited HER. In particular, the methanol-enabled electrolyte presents a record high NRR FE of 75.9 ± 4.1% and ammonia yield rate of 262.5 ± 7.3 micrograms per hour per milligram of catalyst (FeOOH/CNTs), indicative of ∼8-fold enhancements compared with that in conventional aqueous electrolytes and the universality over the other catalysts.
中文翻译:

甲醇介导的氨电合成
由于竞争性析氢反应(HER),电化学氮还原反应(NRR)的氨的发展目前面临着法拉第效率(FE)低的困境。质子供体在电极-电解质界面的质子供体能力在抑制 HER 进而提高 NRR 的选择性方面起着关键作用。根据醇和水之间质子供给能力的内在差异,我们在此展示了一种创新性良好控制的醇-水电解质系统,以调节电极-电解质界面处的局部质子浓度和微环境,其中可用性和解离过程水的吸收可以被大大限制,伴随着扩大的电化学窗口和抑制 HER。特别是,
更新日期:2021-11-12
中文翻译:

甲醇介导的氨电合成
由于竞争性析氢反应(HER),电化学氮还原反应(NRR)的氨的发展目前面临着法拉第效率(FE)低的困境。质子供体在电极-电解质界面的质子供体能力在抑制 HER 进而提高 NRR 的选择性方面起着关键作用。根据醇和水之间质子供给能力的内在差异,我们在此展示了一种创新性良好控制的醇-水电解质系统,以调节电极-电解质界面处的局部质子浓度和微环境,其中可用性和解离过程水的吸收可以被大大限制,伴随着扩大的电化学窗口和抑制 HER。特别是,



























 京公网安备 11010802027423号
京公网安备 11010802027423号