当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonia Synthesis at Ambient Conditions via Electrochemical Atomic Hydrogen Permeation
ACS Energy Letters ( IF 19.3 ) Pub Date : 2021-10-10 , DOI: 10.1021/acsenergylett.1c01568 Davide Ripepi 1 , Riccardo Zaffaroni 1 , Herman Schreuders 1 , Bart Boshuizen 1 , Fokko M Mulder 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2021-10-10 , DOI: 10.1021/acsenergylett.1c01568 Davide Ripepi 1 , Riccardo Zaffaroni 1 , Herman Schreuders 1 , Bart Boshuizen 1 , Fokko M Mulder 1
Affiliation
|
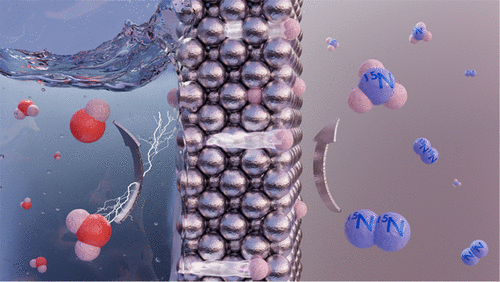
Direct electrochemical nitrogen reduction holds the promise of enabling the production of carbon emission-free ammonia, which is an important intermediate in the fertilizer industry and a potential green energy carrier. Here we show a strategy for ambient condition ammonia synthesis using a hydrogen permeable nickel membrane/electrode that spatially separates the electrolyte and hydrogen reduction side from the dinitrogen activation and hydrogenation sites. Gaseous ammonia is produced catalytically in the absence of electrolyte via hydrogenation of adsorbed nitrogen by electrochemically permeating atomic hydrogen from water reduction. Dinitrogen activation at the polycrystalline nickel surface is confirmed with 15N2 isotope labeling experiments, and it is attributed to a Mars–van Krevelen mechanism enabled by the formation of N-vacancies upon hydrogenation of surface nitrides. We further show that gaseous hydrogen does not hydrogenate the adsorbed nitrogen, strengthening the benefit of having an atomic hydrogen permeable electrode. The proposed approach opens new directions toward green ammonia.
中文翻译:

通过电化学原子氢渗透在环境条件下合成氨
直接电化学氮还原有望实现无碳排放氨的生产,氨是化肥行业的重要中间体和潜在的绿色能源载体。在这里,我们展示了一种使用氢渗透镍膜/电极在环境条件下合成氨的策略,该电极在空间上将电解质和氢还原侧与二氮活化和氢化位点分开。在没有电解质的情况下,通过电化学渗透来自水还原的原子氢对吸附的氮进行氢化,从而催化产生气态氨。用15 N 2确认多晶镍表面的二氮活化同位素标记实验,这归因于通过表面氮化物氢化时形成 N 空位而实现的 Mars-van Krevelen 机制。我们进一步表明,气态氢不会使吸附的氮氢化,从而增强了具有原子氢渗透电极的好处。所提出的方法为绿色氨开辟了新的方向。
更新日期:2021-11-12
中文翻译:

通过电化学原子氢渗透在环境条件下合成氨
直接电化学氮还原有望实现无碳排放氨的生产,氨是化肥行业的重要中间体和潜在的绿色能源载体。在这里,我们展示了一种使用氢渗透镍膜/电极在环境条件下合成氨的策略,该电极在空间上将电解质和氢还原侧与二氮活化和氢化位点分开。在没有电解质的情况下,通过电化学渗透来自水还原的原子氢对吸附的氮进行氢化,从而催化产生气态氨。用15 N 2确认多晶镍表面的二氮活化同位素标记实验,这归因于通过表面氮化物氢化时形成 N 空位而实现的 Mars-van Krevelen 机制。我们进一步表明,气态氢不会使吸附的氮氢化,从而增强了具有原子氢渗透电极的好处。所提出的方法为绿色氨开辟了新的方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号