European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-10-09 , DOI: 10.1016/j.ejmech.2021.113880 Lama Prema Dhorma 1 , Mahesh K Teli 1 , Bhargav Gupta Nangunuri 1 , Arramshetti Venkanna 1 , Rao Ragam 1 , Arunkranthi Maturi 1 , Anvar Mirzaei 1 , Dang-Khoa Vo 1 , Han-Joo Maeng 1 , Mi-Hyun Kim 1
|
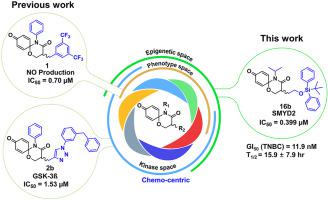
Lysine methyltransferases are important regulators of epigenetic signaling and are emerging as a novel drug target for drug discovery. This work demonstrates the positioning of novel 1,5-oxaza spiroquinone scaffold into selective SET and MYND domain-containing proteins 2 methyltransferases inhibitors. Selectivity of the scaffold was identified by epigenetic target screening followed by SAR study for the scaffold. The optimization was performed iteratively by two-step optimization consisting of iterative synthesis and computational studies (docking, metadynamics simulations). Computational binding studies guided the important interactions of the spiro[5.5]undeca scaffold in pocket 1 and Lysine channel and suggested extension of tail length for the improvement of potency (IC50: up to 399 nM). The effective performance of cell proliferation assay for chosen compounds (IC50: up to 11.9 nM) led to further evaluation in xenograft assay. The potent compound 24 demonstrated desirable in vivo efficacy with growth inhibition rate of 77.7% (4 fold decrease of tumor weight and 3 fold decrease of tumor volume). Moreover, mirosomal assay and pharmacokinetic profile suggested further developability of this scaffold through the identification of major metabolites (dealkylation at silyl group, reversible hydration product, the absence of toxic quinone fragments) and enough exposure of the testing compound 24 in plasma. Such spiro[5.5]undeca framework or ring system was neither been reported nor suggested as a modulator of methyltransferases. The chemo-centric target positioning and structural novelty can lead to potential pharmacological benefit.
中文翻译:

在表观遗传空间中将前所未有的 1,5-氧杂螺醌支架定位到 SMYD2 抑制剂中
赖氨酸甲基转移酶是表观遗传信号的重要调节剂,并且正在成为药物发现的新药物靶点。这项工作证明了将新型 1,5-恶氮螺醌支架定位到选择性 SET 和 MYND 结构域的蛋白质 2 甲基转移酶抑制剂中。支架的选择性通过表观遗传靶点筛选确定,然后对支架进行 SAR 研究。通过由迭代合成和计算研究(对接、元动力学模拟)组成的两步优化迭代地执行优化。计算结合研究指导了口袋 1 中的螺[5.5]undeca 支架和赖氨酸通道的重要相互作用,并建议延长尾长以提高效力 (IC 50: 高达 399 nM)。所选化合物的细胞增殖试验的有效性能(IC 50:高达 11.9 nM)导致异种移植试验的进一步评估。强效化合物24表现出理想的体内功效,生长抑制率为 77.7%(肿瘤重量减少 4 倍,肿瘤体积减少 3 倍)。此外,通过鉴定主要代谢物(甲硅烷基脱烷基化、可逆水合产物、无毒性醌片段)和充分暴露测试化合物24,微粒体测定和药代动力学特征表明该支架的进一步可开发性在血浆中。这种螺[5.5]undeca框架或环系统既没有报道也没有建议作为甲基转移酶的调节剂。以化学为中心的靶点定位和结构新颖性可以带来潜在的药理益处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号