当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antifungal Exploration of Quinoline Derivatives against Phytopathogenic Fungi Inspired by Quinine Alkaloids
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-10-08 , DOI: 10.1021/acs.jafc.1c05677 Yong-Jia Chen 1 , Kun-Yuan Ma 1 , Sha-Sha Du 1 , Zhi-Jun Zhang 1 , Tian-Lin Wu 1 , Yu Sun 1 , Ying-Qian Liu 1 , Xiao-Dan Yin 1 , Rui Zhou 1 , Yin-Fang Yan 1 , Ren-Xuan Wang 1 , Ying-Hui He 1 , Qing-Ru Chu 1 , Chen Tang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-10-08 , DOI: 10.1021/acs.jafc.1c05677 Yong-Jia Chen 1 , Kun-Yuan Ma 1 , Sha-Sha Du 1 , Zhi-Jun Zhang 1 , Tian-Lin Wu 1 , Yu Sun 1 , Ying-Qian Liu 1 , Xiao-Dan Yin 1 , Rui Zhou 1 , Yin-Fang Yan 1 , Ren-Xuan Wang 1 , Ying-Hui He 1 , Qing-Ru Chu 1 , Chen Tang 1
Affiliation
|
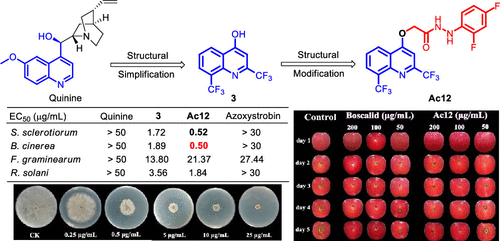
Enlightened from our previous work of structural simplification of quinine and innovative application of natural products against phytopathogenic fungi, lead structure 2,8-bis(trifluoromethyl)-4-quinolinol (3) was selected to be a candidate and its diversified design, synthesis, and antifungal evaluation were carried out. All of the synthesized compounds Aa1–Db1 were evaluated for their antifungal activity against four agriculturally important fungi, Botrytis cinerea, Fusarium graminearum, Rhizoctonia solani, and Sclerotinia sclerotiorum. Results showed that compounds Ac3, Ac4, Ac7, Ac9, Ac12, Bb1, Bb10, Bb11, Bb13, Cb1. and Cb3 exhibited a good antifungal effect, especially Ac12 had the most potent activity with EC50 values of 0.52 and 0.50 μg/mL against S. sclerotiorum and B. cinerea, respectively, which were more potent than those of the lead compound 3 (1.72 and 1.89 μg/mL) and commercial fungicides azoxystrobin (both >30 μg/mL) and 8-hydroxyquinoline (2.12 and 5.28 μg/mL). Moreover, compound Ac12 displayed excellent in vivo antifungal activity, which was comparable in activity to the commercial fungicide boscalid. The preliminary mechanism revealed that compound Ac12 might cause an abnormal morphology of cell membranes, an increase in membrane permeability, and release of cellular contents. These results indicated that compound Ac12 displayed superior in vitro and in vivo fungicidal activities and could be a potential fungicidal candidate against plant fungal diseases.
中文翻译:

奎宁生物碱启发的喹啉衍生物抗植物病原真菌的抗真菌探索
受我们之前奎宁结构简化和天然产物在抗植物病原真菌中的创新应用的启发,选择先导结构 2,8-双(三氟甲基)-4-羟基喹啉(3)作为候选物,其多样化的设计、合成、并进行了抗真菌评估。评估了所有合成的化合物Aa1-Db1对四种农业上重要的真菌,灰葡萄孢、禾谷镰刀菌、立枯丝核菌和核盘菌的抗真菌活性。结果表明化合物Ac3、Ac4、Ac7、Ac9, Ac12 , Bb1 , Bb10 , Bb11 , Bb13 , Cb1。Cb3和Cb3表现出良好的抗真菌作用,尤其是Ac12具有最强的活性,EC 50值分别为 0.52 和 0.50 μg/mL,对S. sclerotiorum和B. cinerea 的抑制作用比先导化合物3 (1.72和 1.89 μg/mL)和商业杀菌剂嘧菌酯(均 >30 μg/mL)和 8-羟基喹啉(2.12 和 5.28 μg/mL)。此外,化合物Ac12在体内表现出色抗真菌活性,其活性与商业杀菌剂啶酰菌胺相当。初步机制表明,化合物Ac12可能导致细胞膜形态异常、膜通透性增加和细胞内容物释放。这些结果表明化合物Ac12在体外和体内表现出优异的杀真菌活性,并且可能是对抗植物真菌病害的潜在杀真菌候选物。
更新日期:2021-10-20
中文翻译:

奎宁生物碱启发的喹啉衍生物抗植物病原真菌的抗真菌探索
受我们之前奎宁结构简化和天然产物在抗植物病原真菌中的创新应用的启发,选择先导结构 2,8-双(三氟甲基)-4-羟基喹啉(3)作为候选物,其多样化的设计、合成、并进行了抗真菌评估。评估了所有合成的化合物Aa1-Db1对四种农业上重要的真菌,灰葡萄孢、禾谷镰刀菌、立枯丝核菌和核盘菌的抗真菌活性。结果表明化合物Ac3、Ac4、Ac7、Ac9, Ac12 , Bb1 , Bb10 , Bb11 , Bb13 , Cb1。Cb3和Cb3表现出良好的抗真菌作用,尤其是Ac12具有最强的活性,EC 50值分别为 0.52 和 0.50 μg/mL,对S. sclerotiorum和B. cinerea 的抑制作用比先导化合物3 (1.72和 1.89 μg/mL)和商业杀菌剂嘧菌酯(均 >30 μg/mL)和 8-羟基喹啉(2.12 和 5.28 μg/mL)。此外,化合物Ac12在体内表现出色抗真菌活性,其活性与商业杀菌剂啶酰菌胺相当。初步机制表明,化合物Ac12可能导致细胞膜形态异常、膜通透性增加和细胞内容物释放。这些结果表明化合物Ac12在体外和体内表现出优异的杀真菌活性,并且可能是对抗植物真菌病害的潜在杀真菌候选物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号