Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2021-10-08 , DOI: 10.1016/j.psep.2021.10.001 Erjun Zhang 1 , Kanggen Zhou 1 , Xuekai Zhang 2 , Yehuizi Wu 1 , Jiajian Liu 1 , Wei Chen 1 , Changhong Peng 1
|
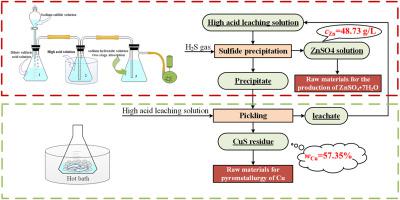
Herein, a new approach for direct separation of Cu and Zn from high acid leaching solution was proposed. The approach includes two steps, Cu and part of Zn first precipitated with H2S, then the Zn in the residue was re-leached using the original leaching solution. Thermodynamics calculation indicates that CuS and As2S3 will generate even the acid concentration is over 8 mol/L, while ZnS is easier to dissolve in acid solution. Therefore, it is possible to selective separate Cu and Zn using H2S under high acid concentration. Key parameters which will affecting the precipitation of Cu and the pickling of Zn are systematically investigated. Under the optimal conditions, the precipitation efficiency of Cu can reach over 99%, and the loss of Zn was only 3.15%. Moreover, more than 96% of As in the leaching solution will also be precipitated. The concentrations of Cu, As, and Zn in the leachate are 0.0722, 0.0893, 48.73 g/L, respectively. The content of Cu in the residue can reach over 57% which could be used for extraction of Cu. XRD results showed that Cu and As were existed as CuS and As2S3 in the residue.
中文翻译:

硫化物沉淀酸洗法从铜粉高酸浸出液中选择性分离铜和锌
在此,提出了一种从强酸浸出液中直接分离铜和锌的新方法。该方法包括两个步骤,首先用H 2 S沉淀Cu和部分Zn ,然后使用原始浸出液重新浸出残留物中的Zn。热力学计算表明,即使酸浓度超过8 mol/L,也会生成CuS和As 2 S 3,而ZnS在酸溶液中更容易溶解。因此,可以使用 H 2选择性分离 Cu 和 ZnS 在高酸浓度下。系统研究了影响Cu析出和Zn酸洗的关键参数。在最佳条件下,Cu的析出效率可达99%以上,Zn的损失仅为3.15%。此外,浸出液中96%以上的As也会析出。浸出液中Cu、As、Zn的浓度分别为0.0722、0.0893、48.73 g/L。残渣中铜含量可达57%以上,可用于提取铜。XRD结果表明Cu和As在残留物中以CuS和As 2 S 3 的形式存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号