当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Genetically Encoded Benzoyllysines Serve as Versatile Probes for Interrogating Histone Benzoylation and Interactions in Living Cells
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-10-07 , DOI: 10.1021/acschembio.1c00614 Hongtao Tian 1, 2 , Jiale Yang 1, 2 , An-Di Guo 1, 2 , Yu Ran 3 , Yun-Zhi Yang 1 , Bing Yang 3 , Ruimin Huang 1, 2 , Haiming Liu 4 , Xiao-Hua Chen 1, 2, 5
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-10-07 , DOI: 10.1021/acschembio.1c00614 Hongtao Tian 1, 2 , Jiale Yang 1, 2 , An-Di Guo 1, 2 , Yu Ran 3 , Yun-Zhi Yang 1 , Bing Yang 3 , Ruimin Huang 1, 2 , Haiming Liu 4 , Xiao-Hua Chen 1, 2, 5
Affiliation
|
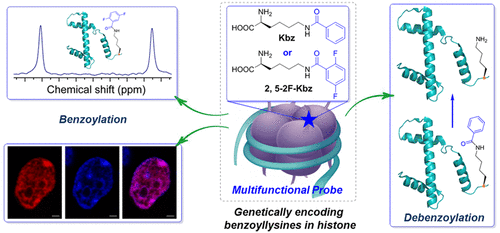
Histone posttranslational modifications (PTMs) are vital epigenetic regulators in many fundamental cell signaling pathways and diverse biological processes. Histone lysine benzoylation is a recently identified epigenetic mark associated with active transcription; however, it remains to be explored. Herein, we first report the genetic encoding of benzoyllysine and fluorinated benzoyllysines into full-length histone proteins in a site-specific manner in live cells, based on our rationally designed synthetase and fine-integrated fluorine element into benzoyllysines. The incorporated unnatural amino acids integrating unique features were demonstrated as versatile probes for investigating histone benzoylation under biological environments, conferring multiplex signals such as 19F NMR spectra with chemical clarity and fluorescence signals for benzoylation. Moreover, the site specifically incorporated lysine benzoylation within native full-length histone proteins revealed distinct dynamics of debenzoylation in the presence of debenzoylase sirtuin 2 (SIRT2). Our developed strategy for genetic encoding of benzoyllysines offers a general and novel approach to gain insights into interactions of site-specific histone benzoylation modifications with interactomes and molecular mechanisms in physiological settings, which could not be accessible with fragment histone peptides. This versatile chemical tool enables a direct and new avenue to explore benzoylation, interactions, and histone epigenetics, which will provide broad utilities in chemical biology, protein science, and basic biology research.
中文翻译:

基因编码的苯甲酰赖氨酸可用作研究活细胞中组蛋白苯甲酰化和相互作用的多功能探针
组蛋白翻译后修饰 (PTM) 是许多基本细胞信号通路和多种生物过程中重要的表观遗传调节剂。组蛋白赖氨酸苯甲酰化是最近发现的与活性转录相关的表观遗传标记。但是,仍有待探索。在这里,我们首先报告了在活细胞中以位点特异性方式将苯甲酰赖氨酸和氟化苯甲酰赖氨酸遗传编码为全长组蛋白,基于我们合理设计的合成酶和将氟元素精细整合到苯甲酰赖氨酸中。整合的非天然氨基酸整合了独特的特征,被证明是用于研究生物环境下组蛋白苯甲酰化的多功能探针,可提供多重信号,例如19F NMR 光谱具有化学透明度和苯甲酰化的荧光信号。此外,该位点在天然全长组蛋白中特异性地掺入了赖氨酸苯甲酰化,揭示了在去苯甲酰酶 sirtuin 2 (SIRT2) 存在下不同的去苯甲酰化动力学。我们开发的苯甲酰赖氨酸基因编码策略提供了一种通用且新颖的方法,可以深入了解位点特异性组蛋白苯甲酰化修饰与相互作用组的相互作用以及生理环境中的分子机制,而片段组蛋白肽无法获得这些信息。这种多功能的化学工具为探索苯甲酰化、相互作用和组蛋白表观遗传学提供了一条直接的新途径,这将为化学生物学、蛋白质科学和基础生物学研究提供广泛的应用。
更新日期:2021-11-19
中文翻译:

基因编码的苯甲酰赖氨酸可用作研究活细胞中组蛋白苯甲酰化和相互作用的多功能探针
组蛋白翻译后修饰 (PTM) 是许多基本细胞信号通路和多种生物过程中重要的表观遗传调节剂。组蛋白赖氨酸苯甲酰化是最近发现的与活性转录相关的表观遗传标记。但是,仍有待探索。在这里,我们首先报告了在活细胞中以位点特异性方式将苯甲酰赖氨酸和氟化苯甲酰赖氨酸遗传编码为全长组蛋白,基于我们合理设计的合成酶和将氟元素精细整合到苯甲酰赖氨酸中。整合的非天然氨基酸整合了独特的特征,被证明是用于研究生物环境下组蛋白苯甲酰化的多功能探针,可提供多重信号,例如19F NMR 光谱具有化学透明度和苯甲酰化的荧光信号。此外,该位点在天然全长组蛋白中特异性地掺入了赖氨酸苯甲酰化,揭示了在去苯甲酰酶 sirtuin 2 (SIRT2) 存在下不同的去苯甲酰化动力学。我们开发的苯甲酰赖氨酸基因编码策略提供了一种通用且新颖的方法,可以深入了解位点特异性组蛋白苯甲酰化修饰与相互作用组的相互作用以及生理环境中的分子机制,而片段组蛋白肽无法获得这些信息。这种多功能的化学工具为探索苯甲酰化、相互作用和组蛋白表观遗传学提供了一条直接的新途径,这将为化学生物学、蛋白质科学和基础生物学研究提供广泛的应用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号