当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
π-Lewis-Base-Catalyzed Asymmetric Vinylogous Umpolung Reactions of Cyclopentadienones and Tropone
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-10-07 , DOI: 10.1002/anie.202111708 Xing-Xing Yang 1 , Ru-Jie Yan 1 , Guang-Yao Ran 1 , Chen Chen 1 , Jing-Fei Yue 1 , Xiao Yan 2 , Qin Ouyang 2 , Wei Du 1 , Ying-Chun Chen 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-10-07 , DOI: 10.1002/anie.202111708 Xing-Xing Yang 1 , Ru-Jie Yan 1 , Guang-Yao Ran 1 , Chen Chen 1 , Jing-Fei Yue 1 , Xiao Yan 2 , Qin Ouyang 2 , Wei Du 1 , Ying-Chun Chen 1, 2
Affiliation
|
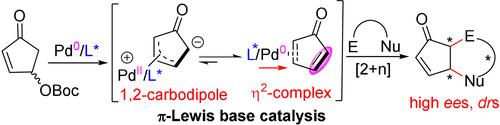
π-Allylpalladium-based 1,2-carbodipoles were generated from the carbonate derivatives of 4-hydroxy-2-cyclopentenones and isomerized in situ to electronically neutral η2-Pd0-cyclopentadienone complexes. These HOMO-raised cyclopentadienones participate, via π-Lewis base activation, in inverse-electron-demand Diels–Alder-type cycloadditions and cascade Morita–Baylis–Hillman- or Rauhut–Currier-type addition/allylic substitution reactions with diverse electrophiles.

中文翻译:

π-刘易斯碱催化的环戊二烯酮和托酮的不对称乙烯基Umpolung反应
π-烯丙基钯基 1,2-碳二极化合物由 4-羟基-2-环戊烯酮的碳酸酯衍生物产生,并原位异构化为电子中性 η 2 -Pd 0 -环戊二烯酮配合物。这些 HOMO 引发的环戊二烯酮通过 π-刘易斯碱活化参与反电子需求 Diels-Alder 型环加成和级联 Morita-Baylis-Hillman 或 Rauhut-Currier 型加成/烯丙基取代反应与不同的亲电子试剂。

更新日期:2021-12-06

中文翻译:

π-刘易斯碱催化的环戊二烯酮和托酮的不对称乙烯基Umpolung反应
π-烯丙基钯基 1,2-碳二极化合物由 4-羟基-2-环戊烯酮的碳酸酯衍生物产生,并原位异构化为电子中性 η 2 -Pd 0 -环戊二烯酮配合物。这些 HOMO 引发的环戊二烯酮通过 π-刘易斯碱活化参与反电子需求 Diels-Alder 型环加成和级联 Morita-Baylis-Hillman 或 Rauhut-Currier 型加成/烯丙基取代反应与不同的亲电子试剂。








































 京公网安备 11010802027423号
京公网安备 11010802027423号