当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
High-Content Phenotypic Screen of a Focused TCAMS Drug Library Identifies Novel Disruptors of the Malaria Parasite Calcium Dynamics
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-10-05 , DOI: 10.1021/acschembio.1c00512 Wanni Chia 1 , Maria G Gomez-Lorenzo 2 , Isabel Castellote 2 , Jie Xin Tong 1 , Rajesh Chandramohanadas 1 , Trang Thi Thu Chu 1 , Wanxiang Shen 3 , Mei Lin Go 3 , Cristina de Cozar 2 , Benigno Crespo 2 , Maria J Almela 2 , Fernando Neria-Serrano 2 , Virginia Franco 2 , Francisco-Javier Gamo 2 , Kevin S W Tan 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-10-05 , DOI: 10.1021/acschembio.1c00512 Wanni Chia 1 , Maria G Gomez-Lorenzo 2 , Isabel Castellote 2 , Jie Xin Tong 1 , Rajesh Chandramohanadas 1 , Trang Thi Thu Chu 1 , Wanxiang Shen 3 , Mei Lin Go 3 , Cristina de Cozar 2 , Benigno Crespo 2 , Maria J Almela 2 , Fernando Neria-Serrano 2 , Virginia Franco 2 , Francisco-Javier Gamo 2 , Kevin S W Tan 1
Affiliation
|
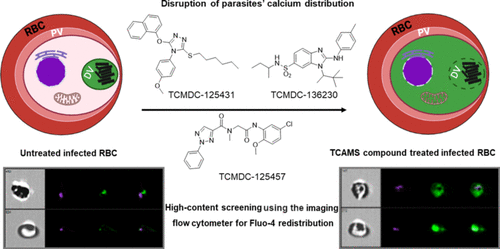
The search for new antimalarial drugs with unexplored mechanisms of action is currently one of the main objectives to combat the resistance already in the clinic. New drugs should target specific mechanisms that once initiated lead inevitably to the parasite’s death and clearance and cause minimal toxicity to the host. One such new mode of action recently characterized is to target the parasite’s calcium dynamics. Disruption of the calcium homeostasis is associated with compromised digestive vacuole membrane integrity and release of its contents, leading to programmed cell death-like features characterized by loss of mitochondrial membrane potential and DNA degradation. Intriguingly, chloroquine (CQ)-treated parasites were previously reported to exhibit such cellular features. Using a high-throughput phenotypic screen, we identified 158 physiological disruptors (hits) of parasite calcium distribution from a small subset of approximately 3000 compounds selected from the GSK TCAMS (Tres Cantos Anti-Malarial Set) compound library. These compounds were then extensively profiled for biological activity against various CQ- and artemisinin-resistant Plasmodium falciparum strains and stages. The hits were also examined for cytotoxicity, speed of antimalarial activity, and their possible inhibitory effects on heme crystallization. Overall, we identified three compounds, TCMDC-136230, -125431, and -125457, which were potent in inducing calcium redistribution but minimally inhibited heme crystallization. Molecular superimposition of the molecules by computational methods identified a common pharmacophore, with the best fit assigned to TCMDC-125457. There were low cytotoxicity or CQ cross-resistance issues for these three compounds. IC50 values of these three compounds were in the low micromolar range. In addition, TCMDC-125457 demonstrated high efficacy when pulsed in a single-dose combination with artesunate against tightly synchronized artemisinin-resistant ring-stage parasites. These results should add new drug options to the current armament of antimalarial drugs as well as provide promising starting points for development of drugs with non-classical modes of action.
中文翻译:

重点 TCAMS 药物库的高内涵表型筛选可识别疟疾寄生虫钙动力学的新型干扰物
寻找具有未探索作用机制的新抗疟疾药物是目前对抗临床耐药性的主要目标之一。新药应针对一旦启动就不可避免地导致寄生虫死亡和清除并对宿主造成最小毒性的特定机制。最近描述的一种新的作用模式是针对寄生虫的钙动力学。钙稳态的破坏与消化液泡膜完整性及其内容物的释放有关,导致以线粒体膜电位丧失和 DNA 降解为特征的程序性细胞死亡样特征。有趣的是,先前报道过氯喹 (CQ) 处理的寄生虫表现出这种细胞特征。使用高通量表型筛选,我们从 GSK TCAMS(Tres Cantos Anti-Malarial Set)化合物库中选择的大约 3000 种化合物的一小部分中鉴定了 158 种寄生虫钙分布的生理干扰物(命中)。然后对这些化合物进行了广泛的分析,以确定其对各种 CQ 和青蒿素抗性的生物活性恶性疟原虫菌株和阶段。还检查了命中的细胞毒性、抗疟活性的速度以及它们对血红素结晶的可能抑制作用。总体而言,我们确定了三种化合物 TCMDC-136230、-125431 和 -125457,它们在诱导钙再分配方面有效,但对血红素结晶的抑制程度最低。通过计算方法对分子进行分子叠加确定了一个常见的药效团,最适合分配给 TCMDC-125457。这三种化合物存在低细胞毒性或 CQ 交叉耐药性问题。集成电路50这三种化合物的值在低微摩尔范围内。此外,TCMDC-125457 与青蒿琥酯单剂量联合脉冲时表现出对紧密同步的青蒿素抗性环状寄生虫的高效能。这些结果应该为目前的抗疟药物增加新的药物选择,并为开发具有非经典作用模式的药物提供有希望的起点。
更新日期:2021-11-19
中文翻译:

重点 TCAMS 药物库的高内涵表型筛选可识别疟疾寄生虫钙动力学的新型干扰物
寻找具有未探索作用机制的新抗疟疾药物是目前对抗临床耐药性的主要目标之一。新药应针对一旦启动就不可避免地导致寄生虫死亡和清除并对宿主造成最小毒性的特定机制。最近描述的一种新的作用模式是针对寄生虫的钙动力学。钙稳态的破坏与消化液泡膜完整性及其内容物的释放有关,导致以线粒体膜电位丧失和 DNA 降解为特征的程序性细胞死亡样特征。有趣的是,先前报道过氯喹 (CQ) 处理的寄生虫表现出这种细胞特征。使用高通量表型筛选,我们从 GSK TCAMS(Tres Cantos Anti-Malarial Set)化合物库中选择的大约 3000 种化合物的一小部分中鉴定了 158 种寄生虫钙分布的生理干扰物(命中)。然后对这些化合物进行了广泛的分析,以确定其对各种 CQ 和青蒿素抗性的生物活性恶性疟原虫菌株和阶段。还检查了命中的细胞毒性、抗疟活性的速度以及它们对血红素结晶的可能抑制作用。总体而言,我们确定了三种化合物 TCMDC-136230、-125431 和 -125457,它们在诱导钙再分配方面有效,但对血红素结晶的抑制程度最低。通过计算方法对分子进行分子叠加确定了一个常见的药效团,最适合分配给 TCMDC-125457。这三种化合物存在低细胞毒性或 CQ 交叉耐药性问题。集成电路50这三种化合物的值在低微摩尔范围内。此外,TCMDC-125457 与青蒿琥酯单剂量联合脉冲时表现出对紧密同步的青蒿素抗性环状寄生虫的高效能。这些结果应该为目前的抗疟药物增加新的药物选择,并为开发具有非经典作用模式的药物提供有希望的起点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号