当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Double Asymmetric Hydrogenation of α-Iminoketones: Facile Synthesis of Enantiopure Vicinal Amino Alcohols
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-05 , DOI: 10.1021/acscatal.1c03635 Jiang Wang 1, 2 , Xin Lin 1, 3 , Pan-Lin Shao 1, 3 , Jingyuan Song 1 , Jialin Wen 1, 4 , Xumu Zhang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-05 , DOI: 10.1021/acscatal.1c03635 Jiang Wang 1, 2 , Xin Lin 1, 3 , Pan-Lin Shao 1, 3 , Jingyuan Song 1 , Jialin Wen 1, 4 , Xumu Zhang 1
Affiliation
|
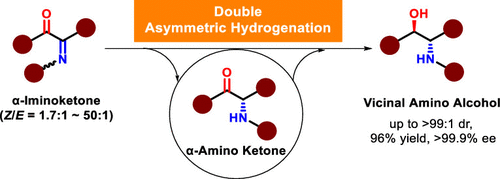
This study presents an Rh/DuanPhos-catalyzed double asymmetric hydrogenation of α-iminoketones for accessing chiral vicinal amino alcohols, which are privileged motifs in pharmaceuticals, agrochemicals, fine chemicals, chiral auxiliaries, organocatalysts, etc. Compared with existing methods, this methodology has the following advantages, such as one-pot operation, high efficiency, operational simplicity, limited waste, broad reaction scope, and high yields (90 to 96%) and stereoselectivities (up to >99:1 dr; >99.9% ee). In addition, the mechanism of the transformation was revealed to be a stepwise reaction by isolating and analyzing reaction intermediates.
中文翻译:

α-亚氨基酮的双不对称氢化:对映纯邻氨基醇的简便合成
本研究介绍了 Rh/DuanPhos 催化的 α-亚氨基酮的双不对称氢化,以获取手性邻氨基醇,这是药物、农用化学品、精细化学品、手性助剂、有机催化剂等中的特权基序。与现有方法相比,该方法具有具有一锅操作、效率高、操作简单、浪费少、反应范围广、收率高(90~96%)和立体选择性(高达>99:1 dr;>99.9% ee)等优点。此外,通过分离和分析反应中间体,揭示了转化的机制是逐步反应。
更新日期:2021-10-15
中文翻译:

α-亚氨基酮的双不对称氢化:对映纯邻氨基醇的简便合成
本研究介绍了 Rh/DuanPhos 催化的 α-亚氨基酮的双不对称氢化,以获取手性邻氨基醇,这是药物、农用化学品、精细化学品、手性助剂、有机催化剂等中的特权基序。与现有方法相比,该方法具有具有一锅操作、效率高、操作简单、浪费少、反应范围广、收率高(90~96%)和立体选择性(高达>99:1 dr;>99.9% ee)等优点。此外,通过分离和分析反应中间体,揭示了转化的机制是逐步反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号