当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Chemo- and Enantioselective Transformations of gem-Diborylalkanes and (Diborylmethyl)metallic Species
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-10-06 , DOI: 10.1021/acs.accounts.1c00455 Yeosan Lee 1 , Seungcheol Han 1 , Seung Hwan Cho 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-10-06 , DOI: 10.1021/acs.accounts.1c00455 Yeosan Lee 1 , Seungcheol Han 1 , Seung Hwan Cho 1
Affiliation
|
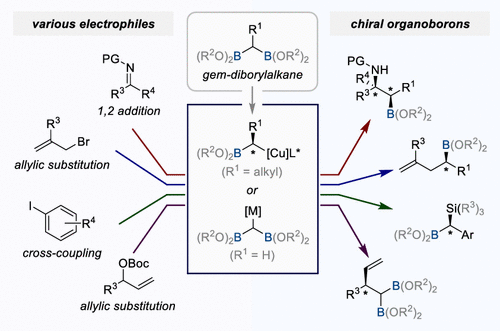
Chemo- and stereoselective transformations of polyborylalkanes are powerful and efficient methods to access optically active molecules with greater complexity and diversity through programmed synthetic design. Among the various polyborylalkanes, gem-diborylalkanes have attracted much attention in organic chemistry as versatile synthetic handles. The notable advantage of gem-diborylalkanes lies in their ability to generate two key intermediates, α-borylalkyl anions and (gem-diborylalkyl) anions. These two different intermediates can be applied to various enantioselective reactions to rapidly access a diverse set of enantioenriched organoboron compounds, which can be further manipulated to generate various chiral molecule libraries via stereospecific C(sp3)–B bond transformations.
中文翻译:

gem-Diborylalkanes 和 (Diborylmethyl) 金属物种的催化化学和对映选择性转化
聚硼烷烃的化学和立体选择性转化是通过程序化合成设计获得具有更大复杂性和多样性的光学活性分子的强大而有效的方法。在各种聚硼烷烃中,宝石二硼烷烃作为多功能合成手柄在有机化学中引起了广泛关注。gem- diborylalkanes的显着优势在于它们能够生成两个关键中间体,α-borylalkane 阴离子和 ( gem- diborylalk ) 阴离子。这两种不同的中间体可以应用于各种对映选择性反应,以快速访问一组不同的对映体富集的有机硼化合物,这些化合物可以通过立体有择的 C(sp3 )–B 键转换。
更新日期:2021-10-19
中文翻译:

gem-Diborylalkanes 和 (Diborylmethyl) 金属物种的催化化学和对映选择性转化
聚硼烷烃的化学和立体选择性转化是通过程序化合成设计获得具有更大复杂性和多样性的光学活性分子的强大而有效的方法。在各种聚硼烷烃中,宝石二硼烷烃作为多功能合成手柄在有机化学中引起了广泛关注。gem- diborylalkanes的显着优势在于它们能够生成两个关键中间体,α-borylalkane 阴离子和 ( gem- diborylalk ) 阴离子。这两种不同的中间体可以应用于各种对映选择性反应,以快速访问一组不同的对映体富集的有机硼化合物,这些化合物可以通过立体有择的 C(sp3 )–B 键转换。











































 京公网安备 11010802027423号
京公网安备 11010802027423号