当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosting eco-friendly hydrogen generation by urea-assisted water electrolysis using spinel M2GeO4 (M = Fe, Co) as an active electrocatalyst
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1en00529d Hyeonuk Choi 1, 2, 3 , Subramani Surendran 1, 2, 3 , Dohun Kim 1, 2, 3 , Yoongu Lim 1, 2, 3, 4 , Jaehyoung Lim 1, 2, 3 , Jihyun Park 1, 2, 3 , Jung Kyu Kim 5 , Mi-Kyung Han 1, 2, 3 , Uk Sim 1, 2, 3, 4
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1en00529d Hyeonuk Choi 1, 2, 3 , Subramani Surendran 1, 2, 3 , Dohun Kim 1, 2, 3 , Yoongu Lim 1, 2, 3, 4 , Jaehyoung Lim 1, 2, 3 , Jihyun Park 1, 2, 3 , Jung Kyu Kim 5 , Mi-Kyung Han 1, 2, 3 , Uk Sim 1, 2, 3, 4
Affiliation
|
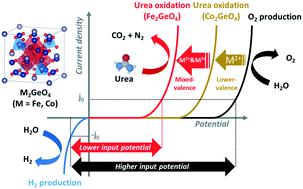
To enhance the efficiency of hydrogen production, bimetallic oxides with spinel structures, M2GeO4 (M = Fe, Co), were synthesized via a facile one-pot hydrothermal method and were used as electrocatalysts for urea-assisted water electrolysis. In alkaline electrolyte with urea, Fe2GeO4, which was used as the anode in the electrolysis cell, exhibited a lower potential (1.53 V (vs. RHE)) and a smaller Tafel slope (76 mV dec−1) Co2GeO4 (1.65 V (vs. RHE), 79 mV dec−1), indicating that Fe2GeO4 reduced the overall input potential to produce H2. The superior performance of Fe2GeO4 in the urea-added water electrolysis was attributed to the higher oxidation state of its metal cations, larger electrochemical active surface area, and lower charge transfer resistance than those of Co2GeO4. Hence, Fe2GeO4 showed 5.49 times higher H2 peak intensity than Co2GeO4, indicating higher efficiency of H2 production.
中文翻译:

使用尖晶石 M2GeO4 (M = Fe, Co) 作为活性电催化剂通过尿素辅助水电解促进环保制氢
为了提高制氢效率,通过简便的一锅水热法合成了具有尖晶石结构的双金属氧化物 M 2 GeO 4 (M = Fe, Co),并将其用作尿素辅助水电解的电催化剂。在含尿素的碱性电解液中,用作电解槽阳极的Fe 2 GeO 4表现出较低的电位(1.53 V(相对于RHE))和较小的 Tafel 斜率(76 mV dec -1)Co 2 GeO 4 (1.65 V ( vs. RHE), 79 mV dec -1 ),表明 Fe 2 GeO 4降低了产生 H 2的总投入潜力。Fe 2 GeO 4在尿素加水电解中的优越性能归因于其金属阳离子的更高氧化态、更大的电化学活性表面积和比Co 2 GeO 4更低的电荷转移电阻。因此,Fe 2 GeO 4显示出比Co 2 GeO 4高5.49 倍的H 2峰强度,表明H 2生产效率更高。
更新日期:2021-10-06
中文翻译:

使用尖晶石 M2GeO4 (M = Fe, Co) 作为活性电催化剂通过尿素辅助水电解促进环保制氢
为了提高制氢效率,通过简便的一锅水热法合成了具有尖晶石结构的双金属氧化物 M 2 GeO 4 (M = Fe, Co),并将其用作尿素辅助水电解的电催化剂。在含尿素的碱性电解液中,用作电解槽阳极的Fe 2 GeO 4表现出较低的电位(1.53 V(相对于RHE))和较小的 Tafel 斜率(76 mV dec -1)Co 2 GeO 4 (1.65 V ( vs. RHE), 79 mV dec -1 ),表明 Fe 2 GeO 4降低了产生 H 2的总投入潜力。Fe 2 GeO 4在尿素加水电解中的优越性能归因于其金属阳离子的更高氧化态、更大的电化学活性表面积和比Co 2 GeO 4更低的电荷转移电阻。因此,Fe 2 GeO 4显示出比Co 2 GeO 4高5.49 倍的H 2峰强度,表明H 2生产效率更高。









































 京公网安备 11010802027423号
京公网安备 11010802027423号