Journal of Advanced Research ( IF 10.7 ) Pub Date : 2021-10-05 , DOI: 10.1016/j.jare.2021.10.001 Hui Yang 1, 2 , Yiren Hu 1, 3 , Mingzhe Weng 1, 4 , Xiaocen Liu 1, 5 , Ping Wan 1, 6 , Ye Hu 7 , Mingzhe Ma 1, 8, 9 , Yan Zhang 1, 10 , Hongping Xia 11 , Kun Lv 1, 2
|
Introduction
Tumors are usually refractory to anti-cancer therapeutics under hypoxic conditions. However, the underlying molecular mechanism remains to be elucidated.
Objectives
Our study intended to identify hypoxia inducible lncRNAs and their biological function in gastric cancer (GC).
Methods
Differentially expressed lncRNAs were determined by microarray analysis between GC cells exposed to hypoxia (1% O2) and normoxia (21% O2) for 24 h. The expression level of CBSLR was manipulated in several GC cell lines to perform molecular and biological analyses both in vitro and in vivo.
Results
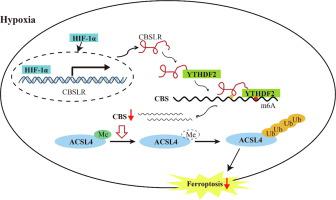
We identified a hypoxia-induced lncRNA-CBSLR that protected GC cells from ferroptosis, leading to chem-resistance. Mechanically, CBSLR interacted with YTHDF2 to form a CBSLR/YTHDF2/CBS signaling axis that decreased the stability of CBS mRNA by enhancing the binding of YTHDF2 with the m6A-modified coding sequence (CDS) of CBS mRNA. Furthermore, under decreased CBS levels, the methylation of the ACSL4 protein was reduced, leading to protein polyubiquitination and degradation of ACSL4. This, in turn, decreased the pro-ferroptosis phosphatidylethanolamine (PE) (18:0/20:4) and PE (18:0/22:4) content and contributed to ferroptosis resistance. Notably, CBSLR is upregulated, whereas CBS is downregulated in GC tissues compared to matched normal tissues; and GC patients with high CBSLR/low CBS levels have a worse clinical outcome and a poorer response to chemotherapy.
Conclusion
Our study reveals a novel mechanism in how HIF1α/CBSLR modulates ferroptosis/chemoresistance in GC, illuminating potential therapeutic targets for refractory hypoxic tumors.
中文翻译:

缺氧诱导型 lncRNA-CBSLR 通过 m6A-YTHDF2 依赖性调节 CBS 在胃癌中调节铁死亡
介绍
在缺氧条件下,肿瘤通常对抗癌治疗无效。然而,潜在的分子机制仍有待阐明。
目标
我们的研究旨在鉴定缺氧诱导的 lncRNA 及其在胃癌 (GC) 中的生物学功能。
方法
通过微阵列分析在暴露于缺氧(1% O 2)和常氧(21% O 2)24小时的GC细胞之间确定差异表达的lncRNA 。在几个 GC 细胞系中操纵CBSLR的表达水平,以在体外和体内进行分子和生物学分析。
结果
我们鉴定了一种缺氧诱导的 lncRNA- CBSLR,它保护 GC 细胞免于铁死亡,导致化学抗性。在机械上,CBSLR与 YTHDF2 相互作用形成CBSLR /YTHDF2/CBS 信号轴,通过增强 YTHDF2 与 CBS mRNA 的 m6A 修饰编码序列 (CDS) 的结合来降低 CBS mRNA 的稳定性。此外,在 CBS 水平降低的情况下,ACSL4 蛋白的甲基化降低,导致蛋白多泛素化和 ACSL4 降解。这反过来又降低了促铁死亡磷脂酰乙醇胺 (PE) (18:0/20:4) 和 PE (18:0/22:4) 的含量,并有助于抗铁死亡。值得注意的是,CBSLR上调,而与匹配的正常组织相比,CBS 在 GC 组织中下调;高CBSLR /低 CBS 水平的GC 患者临床结果较差,对化疗的反应较差。
结论
我们的研究揭示了 HIF1α/ CBSLR如何调节 GC 中的铁死亡/化学抗性的新机制,为难治性缺氧肿瘤提供了潜在的治疗靶点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号