Water Research ( IF 11.4 ) Pub Date : 2021-10-05 , DOI: 10.1016/j.watres.2021.117738 Tan Meng 1 , Wenjun Sun 2 , Xiao Su 3 , Peizhe Sun 1
|
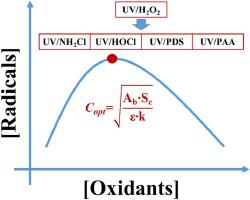
UV-based advanced oxidation processes (AOPs) via photolysis of precursor chemical oxidants have been of interest to numerous researchers over the past several decades due to their capacity to generate highly active radical species and interesting radical chemistry. However, applications of UV-based AOPs have been commonly optimized case by case, due to the lack of theoretical investigations on process optimization, especially on oxidant doses. In this study, a simple equation for UV/H2O2 (•OH as the sole primary reactive species (PRS)) to obtain the theoretical optimal concentration (Copt-theoretical) for H2O2 was derived (Copt-theoretical ). The equation was then validated for its accuracy in the calculation of Copt-theoretical for H2O2 in the UV/H2O2 AOP using a well-established comprehensive kinetic model. A competition kinetics method for the measurement of scavenging capacity (Sc, the unknown parameter for the simple equation) was designed, for which nitrobenzene was employed as the probe compound and tert‑butyl alcohol was introduced as the standard compound. Based on this simple equation, we calculated the Copt-theoretical of 77 environmental water samples and introduced the concept of a practical optimal oxidants dose for the UV/H2O2 AOP, while minimizing the operation costs in engineering applications. Moreover, this study mathematically proved that the simple equation obtained from UV/H2O2 could be successfully extended to other UV-based AOPs, including UV/chlorine, UV/NH2Cl, UV/S2O82−, and UV/peracetic acid. The simple equation of Copt-theoretical derived in this study may not only help to provide instructions for engineering applications, but also point out the ultimate treatment capability of each UV-based AOPs.
中文翻译:

相对于初级自由基浓度,基于紫外线的高级氧化过程中氧化剂的最佳剂量
在过去的几十年中,通过前体化学氧化剂的光解进行的基于紫外线的高级氧化过程 (AOP) 已经引起了众多研究人员的兴趣,因为它们能够产生高活性自由基物种和有趣的自由基化学。然而,由于缺乏对工艺优化,尤其是氧化剂剂量的理论研究,基于紫外线的 AOP 的应用通常会逐个优化。在本研究中,导出了一个简单的 UV/H 2 O 2方程(•OH 作为唯一的主要反应物质(PRS)),以获得H 2 O 2的理论最佳浓度(C opt-理论)(C opt-理论的 )。然后将方程进行了验证其在的计算精度Ç停用理论用于h 2 Ó 2在UV / H 2 ö 2 AOP使用公建立了全面的动力学模型。以硝基苯为探针化合物,以叔丁醇为标准化合物,设计了一种用于测量清除能力(S c,简单方程的未知参数)的竞争动力学方法。基于这个简单的方程,我们计算了77 个环境水样的C光学理论,并引入了 UV/H 2的实用最佳氧化剂剂量的概念。O 2 AOP,同时最大限度地降低工程应用中的运营成本。此外,这项研究在数学上证明了从 UV/H 2 O 2获得的简单方程可以成功地扩展到其他基于 UV 的 AOP,包括 UV/氯、UV/NH 2 Cl、UV/S 2 O 8 2−和紫外线/过乙酸。本研究中推导出的C opt-theoretical的简单方程不仅有助于为工程应用提供指导,而且还指出了每种基于UV的AOP的最终处理能力。










































 京公网安备 11010802027423号
京公网安备 11010802027423号