Chemical & Pharmaceutical Bulletin ( IF 1.7 ) Pub Date : 2021-10-01 , DOI: 10.1248/cpb.c21-00258 Yutaka Matsuda 1 , Brian A Mendelsohn 2
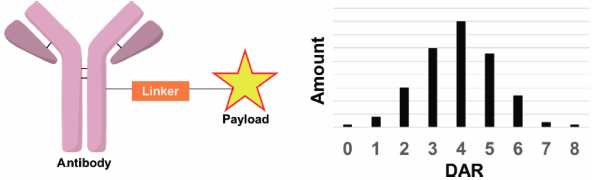
Antibody–drug conjugates (ADCs) are biopharmaceuticals produced by chemically linking small molecules (payloads) to antibodies that possess specific affinity for the target cell. The ADCs currently on the commercially market are the result of a stochastic conjugation of highly-potent payloads to multiple sites on the monoclonal antibody, resulting in a heterogeneous drug–antibody ratio (DAR) and drug distribution. The heterogeneity inherent to ADCs not produced site-specifically may not only be detrimental to the quality of the drug but also is less-desirable from the perspective of regulatory science. An ideal method or unified approach used to measure the DAR for ADCs, a critical aspect of their analysis and characterization, has not yet been established in the ADC field and remains an often-challenging issue for bioanalytical chemists. In this review we describe, compare, and evaluate the characteristics of various DAR determination methods for ADCs featuring recently reported technologies. The future landscape of bioconjugate DAR analysis is also discussed.
 Fullsize Image
Fullsize Image
中文翻译:

抗体-药物偶联物的药物-抗体比测定的最新进展
抗体药物偶联物 (ADC) 是通过将小分子(有效载荷)与对靶细胞具有特定亲和力的抗体化学连接而产生的生物药物。目前商业市场上的 ADC 是高效载荷与单克隆抗体上多个位点随机结合的结果,导致药物抗体比 (DAR) 和药物分布的异质性。ADC 的固有异质性不是特定于位点产生的,不仅可能不利于药物的质量,而且从监管科学的角度来看也不太理想。用于测量 ADC 的 DAR 的理想方法或统一方法是其分析和表征的一个关键方面,尚未在 ADC 领域建立,并且仍然是生物分析化学家经常面临的挑战。在这篇综述中,我们描述、比较和评估了具有最近报道的技术的 ADC 的各种 DAR 确定方法的特征。还讨论了生物共轭 DAR 分析的未来前景。
 全尺寸图像
全尺寸图像



























 京公网安备 11010802027423号
京公网安备 11010802027423号