当前位置:
X-MOL 学术
›
Chem. Pharm. Bull.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
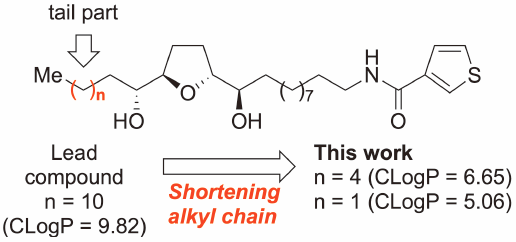
Structure–Activity Relationships of Thiophene Carboxamide Annonaceous Acetogenin Analogs: Shortening the Alkyl Chain in the Tail Part Significantly Affects Their Growth Inhibitory Activity against Human Cancer Cell Lines
Chemical & Pharmaceutical Bulletin ( IF 1.5 ) Pub Date : 2021-10-01 , DOI: 10.1248/cpb.c21-00450 Kaito Ohta 1 , Akinobu Akatsuka 2 , Shingo Dan 2 , Hiroki Iwasaki 1 , Masayuki Yamashita 1 , Naoto Kojima 1
 Fullsize Image
Fullsize Image
中文翻译:

噻吩甲酰胺番荔枝苷类似物的构效关系:缩短尾部的烷基链显着影响它们对人类癌细胞系的生长抑制活性
 全尺寸图像
更新日期:2021-10-21
全尺寸图像
更新日期:2021-10-21
Chemical & Pharmaceutical Bulletin ( IF 1.5 ) Pub Date : 2021-10-01 , DOI: 10.1248/cpb.c21-00450 Kaito Ohta 1 , Akinobu Akatsuka 2 , Shingo Dan 2 , Hiroki Iwasaki 1 , Masayuki Yamashita 1 , Naoto Kojima 1
Affiliation
In a previous study, we found that the thiophene carboxamide solamin analog, which is a mono-tetrahydrofuran annonaceous acetogenin, showed potent antitumor activity through the inhibition of mitochondrial complex I. In this study, we synthesized analogs with short alkyl chains instead of the n-dodecyl group in the tail part. We evaluated their growth inhibitory activities against human cancer cell lines. We found that the alkyl chain in the tail part plays an essential role in their activity.
 Fullsize Image
Fullsize Image
中文翻译:

噻吩甲酰胺番荔枝苷类似物的构效关系:缩短尾部的烷基链显着影响它们对人类癌细胞系的生长抑制活性
在之前的一项研究中,我们发现噻吩甲酰胺醇溶蛋白类似物是一种单四氢呋喃番荔枝素,通过抑制线粒体复合物 I 显示出有效的抗肿瘤活性。在这项研究中,我们合成了具有短烷基链的类似物,而不是n - 尾部的十二烷基。我们评估了它们对人类癌细胞系的生长抑制活性。我们发现尾部的烷基链在它们的活性中起着至关重要的作用。
 全尺寸图像
全尺寸图像











































 京公网安备 11010802027423号
京公网安备 11010802027423号