当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A rush to explore protein–ligand electrostatic interaction energy with Charger
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-10-04 , DOI: 10.1107/s2059798321008433 Vedran Vuković 1 , Theo Leduc 1 , Zoe Jelić-Matošević 2 , Claude Didierjean 1 , Frédérique Favier 1 , Benoît Guillot 1 , Christian Jelsch 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-10-04 , DOI: 10.1107/s2059798321008433 Vedran Vuković 1 , Theo Leduc 1 , Zoe Jelić-Matošević 2 , Claude Didierjean 1 , Frédérique Favier 1 , Benoît Guillot 1 , Christian Jelsch 1
Affiliation
|
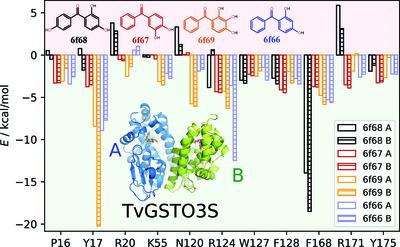
The mutual penetration of electron densities between two interacting molecules complicates the computation of an accurate electrostatic interaction energy based on a pseudo-atom representation of electron densities. The numerical exact potential and multipole moment (nEP/MM) method is time-consuming since it performs a 3D integration to obtain the electrostatic energy at short interaction distances. Nguyen et al. [(2018), Acta Cryst. A74, 524–536] recently reported a fully analytical computation of the electrostatic interaction energy (aEP/MM). This method performs much faster than nEP/MM (up to two orders of magnitude) and remains highly accurate. A new program library, Charger, contains an implementation of the aEP/MM method. Charger has been incorporated into the MoProViewer software. Benchmark tests on a series of small molecules containing only C, H, N and O atoms show the efficiency of Charger in terms of execution time and accuracy. Charger is also powerful in a study of electrostatic symbiosis between a protein and a ligand. It determines reliable protein–ligand interaction energies even when both contain S atoms. It easily estimates the individual contribution of every residue to the total protein–ligand electrostatic binding energy. Glutathione transferase (GST) in complex with a benzophenone ligand was studied due to the availability of both structural and thermodynamic data. The resulting analysis highlights not only the residues that stabilize the ligand but also those that hinder ligand binding from an electrostatic point of view. This offers new perspectives in the search for mutations to improve the interaction between the two partners. A proposed mutation would improve ligand binding to GST by removing an electrostatic obstacle, rather than by the traditional increase in the number of favourable contacts.
中文翻译:

急于用 Charger 探索蛋白质-配体静电相互作用能
两个相互作用分子之间电子密度的相互渗透使基于电子密度的伪原子表示的精确静电相互作用能的计算变得复杂。数值精确电位和多极矩 (nEP/MM) 方法非常耗时,因为它执行 3D 积分以获得短相互作用距离处的静电能量。阮等人。[(2018 年),水晶学报。A 74 , 524–536] 最近报道了对静电相互作用能 (aEP/MM) 的完全分析计算。这种方法的执行速度比 nEP/MM 快得多(最多两个数量级),并且保持高度准确。新的程序库Charger包含 aEP/MM 方法的实现。Charger已集成到MoProViewer软件中。对仅包含 C、H、N 和 O 原子的一系列小分子的基准测试显示了Charger在执行时间和准确性方面的效率。充电器在研究蛋白质和配体之间的静电共生方面也很强大。即使两者都含有 S 原子,它也能确定可靠的蛋白质-配体相互作用能。它很容易估计每个残基对总蛋白质-配体静电结合能的贡献。由于结构和热力学数据的可用性,研究了与二苯甲酮配体复合的谷胱甘肽转移酶 (GST)。结果分析不仅突出了稳定配体的残基,还突出了从静电角度阻碍配体结合的残基。这为寻找突变以改善两个合作伙伴之间的相互作用提供了新的视角。提议的突变将通过消除静电障碍来改善配体与 GST 的结合,
更新日期:2021-10-04
中文翻译:

急于用 Charger 探索蛋白质-配体静电相互作用能
两个相互作用分子之间电子密度的相互渗透使基于电子密度的伪原子表示的精确静电相互作用能的计算变得复杂。数值精确电位和多极矩 (nEP/MM) 方法非常耗时,因为它执行 3D 积分以获得短相互作用距离处的静电能量。阮等人。[(2018 年),水晶学报。A 74 , 524–536] 最近报道了对静电相互作用能 (aEP/MM) 的完全分析计算。这种方法的执行速度比 nEP/MM 快得多(最多两个数量级),并且保持高度准确。新的程序库Charger包含 aEP/MM 方法的实现。Charger已集成到MoProViewer软件中。对仅包含 C、H、N 和 O 原子的一系列小分子的基准测试显示了Charger在执行时间和准确性方面的效率。充电器在研究蛋白质和配体之间的静电共生方面也很强大。即使两者都含有 S 原子,它也能确定可靠的蛋白质-配体相互作用能。它很容易估计每个残基对总蛋白质-配体静电结合能的贡献。由于结构和热力学数据的可用性,研究了与二苯甲酮配体复合的谷胱甘肽转移酶 (GST)。结果分析不仅突出了稳定配体的残基,还突出了从静电角度阻碍配体结合的残基。这为寻找突变以改善两个合作伙伴之间的相互作用提供了新的视角。提议的突变将通过消除静电障碍来改善配体与 GST 的结合,











































 京公网安备 11010802027423号
京公网安备 11010802027423号