当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkyne-Palladium(II)-Catalyzed Living Polymerization of Isocyanides: An Exploration of Diverse Structures and Functions
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-10-03 , DOI: 10.1021/acs.accounts.1c00489 Na Liu 1 , Li Zhou 1 , Zong-Quan Wu 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-10-03 , DOI: 10.1021/acs.accounts.1c00489 Na Liu 1 , Li Zhou 1 , Zong-Quan Wu 1
Affiliation
|
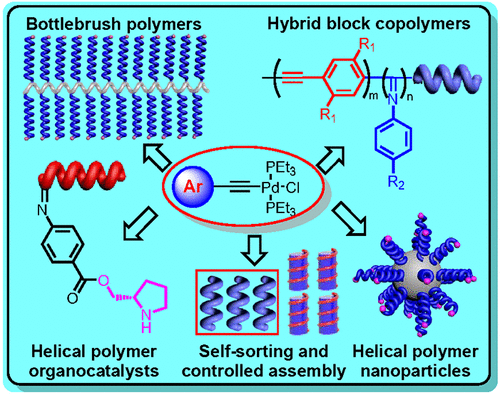
Inspired by the perfect helical structures and the resulting exquisite functions of biomacromolecules, helical polymers have attracted increasing attention in recent years. Polyisocyanide is well known for its distinctive rodlike helical structure and various applications in chiral recognition, enantiomer separation, circularly polarized luminescence, liquid crystallization, and other fields. Although various methods and catalysts for isocyanide polymerization have been reported, the precise synthesis of helical polyisocyanides with controlled molecular weight, low dispersity, and high tacticity remains a formidable challenge. Owing to a limited synthesis strategy, the controlled synthesis of topological polyisocyanides has barely been realized. This Accounts highlights our recent endeavors to explore novel catalysts for the living polymerization of isocyanides. Fortunately, we discovered that alkyne-Pd(II) catalysts could initiate the living polymerization of isocyanides, resulting in helical polyisocyanides with controlled structures, high tacticity, and tunable compositions. These catalysts are applicable to various isocyanide monomers, including alkyl isocyanides, aryl isocyanides, and diisocyanobenzene derivatives. Incorporating chiral bidentate phosphine ligands onto alkyne-Pd(II) complexes formed chiral Pd(II) catalysts, which promoted the asymmetric living polymerization of achiral isocyanide, yielding single left- and right-handed helices with highly optical activities.
中文翻译:

炔-钯 (II) 催化的异氰化物活性聚合:对不同结构和功能的探索
受生物大分子完美的螺旋结构和由此产生的精细功能的启发,螺旋聚合物近年来受到越来越多的关注。聚异氰化物以其独特的棒状螺旋结构和在手性识别、对映异构体分离、圆偏振发光、液体结晶等领域的各种应用而闻名。尽管已经报道了用于异氰化物聚合的各种方法和催化剂,但精确合成具有可控分子量、低分散性和高立构规整度的螺旋聚异氰化物仍然是一项艰巨的挑战。由于有限的合成策略,拓扑聚异氰化物的可控合成几乎没有实现。本报告重点介绍了我们最近为探索用于异氰化物活性聚合的新型催化剂所做的努力。幸运的是,我们发现炔-Pd(II) 催化剂可以引发异氰化物的活性聚合,从而产生具有可控结构、高立构规整度和可调节组成的螺旋聚异氰化物。这些催化剂适用于各种异氰化物单体,包括烷基异氰化物、芳基异氰化物和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。我们发现炔-Pd(II) 催化剂可以引发异氰化物的活性聚合,从而产生具有可控结构、高立构规整度和可调节组成的螺旋聚异氰化物。这些催化剂适用于各种异氰化物单体,包括烷基异氰化物、芳基异氰化物和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。我们发现炔-Pd(II) 催化剂可以引发异氰化物的活性聚合,从而产生具有可控结构、高立构规整度和可调节组成的螺旋聚异氰化物。这些催化剂适用于各种异氰化物单体,包括烷基异氰化物、芳基异氰化物和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。
更新日期:2021-10-19
中文翻译:

炔-钯 (II) 催化的异氰化物活性聚合:对不同结构和功能的探索
受生物大分子完美的螺旋结构和由此产生的精细功能的启发,螺旋聚合物近年来受到越来越多的关注。聚异氰化物以其独特的棒状螺旋结构和在手性识别、对映异构体分离、圆偏振发光、液体结晶等领域的各种应用而闻名。尽管已经报道了用于异氰化物聚合的各种方法和催化剂,但精确合成具有可控分子量、低分散性和高立构规整度的螺旋聚异氰化物仍然是一项艰巨的挑战。由于有限的合成策略,拓扑聚异氰化物的可控合成几乎没有实现。本报告重点介绍了我们最近为探索用于异氰化物活性聚合的新型催化剂所做的努力。幸运的是,我们发现炔-Pd(II) 催化剂可以引发异氰化物的活性聚合,从而产生具有可控结构、高立构规整度和可调节组成的螺旋聚异氰化物。这些催化剂适用于各种异氰化物单体,包括烷基异氰化物、芳基异氰化物和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。我们发现炔-Pd(II) 催化剂可以引发异氰化物的活性聚合,从而产生具有可控结构、高立构规整度和可调节组成的螺旋聚异氰化物。这些催化剂适用于各种异氰化物单体,包括烷基异氰化物、芳基异氰化物和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。我们发现炔-Pd(II) 催化剂可以引发异氰化物的活性聚合,从而产生具有可控结构、高立构规整度和可调节组成的螺旋聚异氰化物。这些催化剂适用于各种异氰化物单体,包括烷基异氰化物、芳基异氰化物和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。和二异氰基苯衍生物。将手性双齿膦配体加入炔-Pd(II)配合物形成手性Pd(II)催化剂,促进非手性异氰化物的不对称活性聚合,产生具有高度光学活性的左旋和右旋单螺旋。









































 京公网安备 11010802027423号
京公网安备 11010802027423号