当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chirality Bias Tissue Homeostasis by Manipulating Immunological Response
Advanced Materials ( IF 27.4 ) Pub Date : 2021-10-03 , DOI: 10.1002/adma.202105136 Shengjie Jiang 1, 2 , Qiang Zeng 1, 2 , Kai Zhao 1, 2 , Jinying Liu 3 , Qiannan Sun 1, 2 , Kang Huang 4 , Ying He 1, 2 , Xuehui Zhang 5 , Hui Wang 4 , Xinghua Shi 4 , Chuanliang Feng 6 , Xuliang Deng 1, 2 , Yan Wei 1, 2
Advanced Materials ( IF 27.4 ) Pub Date : 2021-10-03 , DOI: 10.1002/adma.202105136 Shengjie Jiang 1, 2 , Qiang Zeng 1, 2 , Kai Zhao 1, 2 , Jinying Liu 3 , Qiannan Sun 1, 2 , Kang Huang 4 , Ying He 1, 2 , Xuehui Zhang 5 , Hui Wang 4 , Xinghua Shi 4 , Chuanliang Feng 6 , Xuliang Deng 1, 2 , Yan Wei 1, 2
Affiliation
|
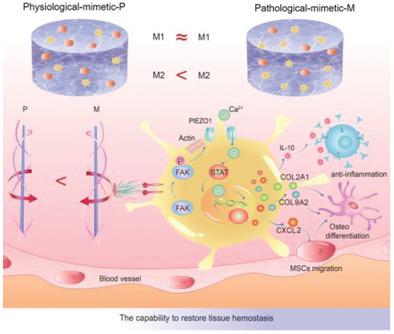
The physiological chirality of extracellular environments is substantially affected by pathological diseases. However, how this stereochemical variation drives host immunity remains poorly understood. Here, it is reported that pathology-mimetic M-nanofibrils—but not physiology-mimetic P-nanofibrils—act as a defense mechanism that helps to restore tissue homeostasis by manipulating immunological response. Quantitative multi-omics in vivo and in vitro shows that M-nanofibrils significantly inhibit inflammation and promote tissue regeneration by upregulating M2 macrophage polarization and downstream immune signaling compared with P-nanofibrils. Molecular analysis and theoretical simulation demonstrate that M-chirality displays higher stereo-affinity to cellular binding, which induces higher cellular contractile stress and activates mechanosensitive ion channel PIEZOl to conduct Ca2+ influx. In turn, the nuclear transfer of STAT is biased by Ca2+ influx to promote M2 polarization. These findings underscore the structural mechanisms of disease, providing design basis for immunotherapy with bionic functional materials.
中文翻译:

通过操纵免疫反应实现手性偏向组织稳态
细胞外环境的生理手性很大程度上受病理疾病的影响。然而,这种立体化学变化如何驱动宿主免疫仍然知之甚少。在这里,据报道,模拟病理学的 M-纳米原纤维 - 但不是模拟生理学的 P-纳米原纤维 - 作为一种防御机制,通过操纵免疫反应帮助恢复组织稳态。体内和体外的定量多组学研究表明,与 P-纳米原纤维相比,M-纳米原纤维通过上调 M2 巨噬细胞极化和下游免疫信号传导显着抑制炎症并促进组织再生。分子分析和理论模拟表明,M-手性对细胞结合表现出更高的立体亲和力,2+涌入。反过来,STAT的核转移被Ca 2+流入偏向以促进M2极化。这些发现强调了疾病的结构机制,为使用仿生功能材料进行免疫治疗提供了设计基础。
更新日期:2021-10-03
中文翻译:

通过操纵免疫反应实现手性偏向组织稳态
细胞外环境的生理手性很大程度上受病理疾病的影响。然而,这种立体化学变化如何驱动宿主免疫仍然知之甚少。在这里,据报道,模拟病理学的 M-纳米原纤维 - 但不是模拟生理学的 P-纳米原纤维 - 作为一种防御机制,通过操纵免疫反应帮助恢复组织稳态。体内和体外的定量多组学研究表明,与 P-纳米原纤维相比,M-纳米原纤维通过上调 M2 巨噬细胞极化和下游免疫信号传导显着抑制炎症并促进组织再生。分子分析和理论模拟表明,M-手性对细胞结合表现出更高的立体亲和力,2+涌入。反过来,STAT的核转移被Ca 2+流入偏向以促进M2极化。这些发现强调了疾病的结构机制,为使用仿生功能材料进行免疫治疗提供了设计基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号