当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computer-Aided Design and Synthesis of a New Class of PEX14 Inhibitors: Substituted 2,3,4,5-Tetrahydrobenzo[F][1,4]oxazepines as Potential New Trypanocidal Agents
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-10-01 , DOI: 10.1021/acs.jcim.1c00472 Roberto Fino 1, 2 , Dominik Lenhart 1, 2, 3, 4 , Vishal C Kalel 5 , Charlotte A Softley 1, 2 , Valeria Napolitano 1, 2 , Ryan Byrne 6 , Wolfgang Schliebs 5 , Maciej Dawidowski 7 , Ralf Erdmann 5 , Michael Sattler 1, 2 , Gisbert Schneider 6 , Oliver Plettenburg 3, 4 , Grzegorz M Popowicz 1, 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-10-01 , DOI: 10.1021/acs.jcim.1c00472 Roberto Fino 1, 2 , Dominik Lenhart 1, 2, 3, 4 , Vishal C Kalel 5 , Charlotte A Softley 1, 2 , Valeria Napolitano 1, 2 , Ryan Byrne 6 , Wolfgang Schliebs 5 , Maciej Dawidowski 7 , Ralf Erdmann 5 , Michael Sattler 1, 2 , Gisbert Schneider 6 , Oliver Plettenburg 3, 4 , Grzegorz M Popowicz 1, 2
Affiliation
|
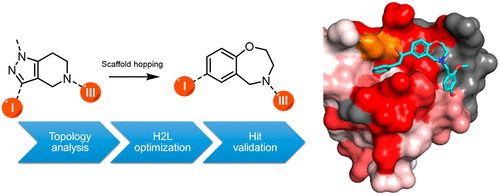
African and American trypanosomiases are estimated to affect several million people across the world, with effective treatments distinctly lacking. New, ideally oral, treatments with higher efficacy against these diseases are desperately needed. Peroxisomal import matrix (PEX) proteins represent a very interesting target for structure- and ligand-based drug design. The PEX5–PEX14 protein–protein interface in particular has been highlighted as a target, with inhibitors shown to disrupt essential cell processes in trypanosomes, leading to cell death. In this work, we present a drug development campaign that utilizes the synergy between structural biology, computer-aided drug design, and medicinal chemistry in the quest to discover and develop new potential compounds to treat trypanosomiasis by targeting the PEX14–PEX5 interaction. Using the structure of the known lead compounds discovered by Dawidowski et al. as the template for a chemically advanced template search (CATS) algorithm, we performed scaffold-hopping to obtain a new class of compounds with trypanocidal activity, based on 2,3,4,5-tetrahydrobenzo[f][1,4]oxazepines chemistry. The initial compounds obtained were taken forward to a first round of hit-to-lead optimization by synthesis of derivatives, which show activities in the range of low- to high-digit micromolar IC50 in the in vitro tests. The NMR measurements confirm binding to PEX14 in solution, while immunofluorescent microscopy indicates disruption of protein import into the glycosomes, indicating that the PEX14–PEX5 protein–protein interface was successfully disrupted. These studies result in development of a novel scaffold for future lead optimization, while ADME testing gives an indication of further areas of improvement in the path from lead molecules toward a new drug active against trypanosomes.
中文翻译:

新型 PEX14 抑制剂的计算机辅助设计和合成:取代的 2,3,4,5-四氢苯[F][1,4] 氧氮杂作为潜在的新型杀锥虫剂
据估计,非洲和美洲锥虫病影响了全世界数百万人,但明显缺乏有效的治疗方法。迫切需要对这些疾病具有更高疗效的新的、理想的口服疗法。过氧化物酶体导入基质 (PEX) 蛋白代表了基于结构和配体的药物设计的一个非常有趣的目标。PEX5-PEX14 蛋白质-蛋白质界面已被特别强调为一个靶点,其抑制剂会破坏锥虫中的基本细胞过程,导致细胞死亡。在这项工作中,我们提出了一项药物开发活动,利用结构生物学、计算机辅助药物设计和药物化学之间的协同作用,通过靶向 PEX14-PEX5 相互作用来发现和开发新的潜在化合物来治疗锥虫病。使用 Dawidowski 等人发现的已知先导化合物的结构。作为化学高级模板搜索 (CATS) 算法的模板,我们基于 2,3,4,5-四氢苯并[f][1,4]氧氮杂,进行了支架跳跃以获得具有杀锥虫活性的一类新化合物化学。获得的初始化合物通过衍生物的合成进行了第一轮的先导优化,显示了低至高数字微摩尔 IC 范围内的活性50在体外试验中。NMR 测量证实与溶液中的 PEX14 结合,而免疫荧光显微镜表明蛋白质进入糖体的中断,表明 PEX14-PEX5 蛋白质-蛋白质界面被成功破坏。这些研究导致开发了一种用于未来铅优化的新型支架,而 ADME 测试表明了从铅分子到对锥虫病有活性的新药物的路径中的进一步改进领域。
更新日期:2021-10-25
中文翻译:

新型 PEX14 抑制剂的计算机辅助设计和合成:取代的 2,3,4,5-四氢苯[F][1,4] 氧氮杂作为潜在的新型杀锥虫剂
据估计,非洲和美洲锥虫病影响了全世界数百万人,但明显缺乏有效的治疗方法。迫切需要对这些疾病具有更高疗效的新的、理想的口服疗法。过氧化物酶体导入基质 (PEX) 蛋白代表了基于结构和配体的药物设计的一个非常有趣的目标。PEX5-PEX14 蛋白质-蛋白质界面已被特别强调为一个靶点,其抑制剂会破坏锥虫中的基本细胞过程,导致细胞死亡。在这项工作中,我们提出了一项药物开发活动,利用结构生物学、计算机辅助药物设计和药物化学之间的协同作用,通过靶向 PEX14-PEX5 相互作用来发现和开发新的潜在化合物来治疗锥虫病。使用 Dawidowski 等人发现的已知先导化合物的结构。作为化学高级模板搜索 (CATS) 算法的模板,我们基于 2,3,4,5-四氢苯并[f][1,4]氧氮杂,进行了支架跳跃以获得具有杀锥虫活性的一类新化合物化学。获得的初始化合物通过衍生物的合成进行了第一轮的先导优化,显示了低至高数字微摩尔 IC 范围内的活性50在体外试验中。NMR 测量证实与溶液中的 PEX14 结合,而免疫荧光显微镜表明蛋白质进入糖体的中断,表明 PEX14-PEX5 蛋白质-蛋白质界面被成功破坏。这些研究导致开发了一种用于未来铅优化的新型支架,而 ADME 测试表明了从铅分子到对锥虫病有活性的新药物的路径中的进一步改进领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号