当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusual Transformations of Strain-Heightened Oxetanes
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-09-30 , DOI: 10.1021/acs.accounts.1c00415 Jason An 1 , Louis P Riel 1 , Amy R Howell 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-09-30 , DOI: 10.1021/acs.accounts.1c00415 Jason An 1 , Louis P Riel 1 , Amy R Howell 1
Affiliation
|
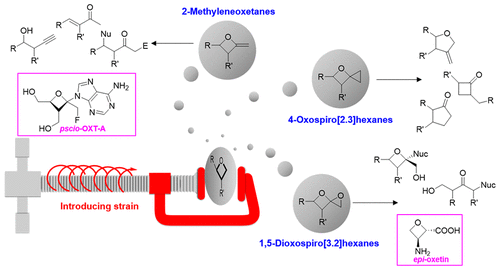
Oxetanes are important motifs for drug discovery and are valuable templates in organic synthesis. Much of their use as synthetic intermediates exploits their inherent strain, often resulting in chain extensions at the expense of the heterocycle. Modifications on the carbon alpha to the oxygen of oxetanes, such as the C═O of β-lactones, extend the modes of reactivity. Nevertheless, the outcomes are still largely predictable. On the other hand, other alpha modifications, such as a ═CH2, a spiro-oxiranyl moiety, or a spiro-cyclopropyl group, increase strain and open pathways not available to simple oxetanes or β-lactones. Methods in generating 2-methyleneoxetanes, 1,5-dioxaspiro[3.2]hexanes, and 4-oxaspiro[2.3]hexanes have been developed by us and others. To date, reactions of these systems have sometimes been predictable, but often the outcomes have been unexpected. This has provided fertile ground for thinking about what controls reactivity and what other reaction pathways might be accessible to these strain-heightened oxetanes.
中文翻译:

应变增加的氧杂环丁烷的不寻常转变
氧杂环丁烷是药物发现的重要基序,并且是有机合成中的宝贵模板。它们作为合成中间体的大部分用途利用了它们固有的应变,通常会导致以杂环为代价的扩链。氧杂环丁烷氧的α碳修饰,例如β-内酯的C=O,扩展了反应模式。尽管如此,结果在很大程度上仍然是可以预测的。另一方面,其他 alpha 修饰,例如 a =CH 2、螺环环氧乙烷基部分或螺环丙基基团可增加简单氧杂环丁烷或 β-内酯无法获得的应变和开放途径。我们和其他人已经开发了产生 2-亚甲基氧杂环丁烷、1,5-二氧杂螺[3.2] 己烷和 4-氧杂螺[2.3] 己烷的方法。迄今为止,这些系统的反应有时是可以预测的,但结果往往是出乎意料的。这为思考什么控制反应性以及这些应变增强的氧杂环丁烷可能有哪些其他反应途径提供了肥沃的土壤。
更新日期:2021-10-19
中文翻译:

应变增加的氧杂环丁烷的不寻常转变
氧杂环丁烷是药物发现的重要基序,并且是有机合成中的宝贵模板。它们作为合成中间体的大部分用途利用了它们固有的应变,通常会导致以杂环为代价的扩链。氧杂环丁烷氧的α碳修饰,例如β-内酯的C=O,扩展了反应模式。尽管如此,结果在很大程度上仍然是可以预测的。另一方面,其他 alpha 修饰,例如 a =CH 2、螺环环氧乙烷基部分或螺环丙基基团可增加简单氧杂环丁烷或 β-内酯无法获得的应变和开放途径。我们和其他人已经开发了产生 2-亚甲基氧杂环丁烷、1,5-二氧杂螺[3.2] 己烷和 4-氧杂螺[2.3] 己烷的方法。迄今为止,这些系统的反应有时是可以预测的,但结果往往是出乎意料的。这为思考什么控制反应性以及这些应变增强的氧杂环丁烷可能有哪些其他反应途径提供了肥沃的土壤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号