当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryo-EM structure of the full-length Lon protease from Thermus thermophilus
FEBS Letters ( IF 3.0 ) Pub Date : 2021-09-30 , DOI: 10.1002/1873-3468.14199 Francesca Coscia 1 , Jan Löwe 1
FEBS Letters ( IF 3.0 ) Pub Date : 2021-09-30 , DOI: 10.1002/1873-3468.14199 Francesca Coscia 1 , Jan Löwe 1
Affiliation
|
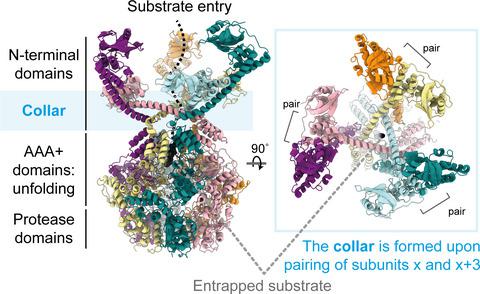
In bacteria, Lon is a large hexameric ATP-dependent protease that targets misfolded and also folded substrates, some of which are involved in cell division and survival of cellular stress. The N-terminal domain of Lon facilitates substrate recognition, but how the domains confer such activity has remained unclear. Here, we report the full-length structure of Lon protease from Thermus thermophilus at 3.9 Å resolution in a substrate-engaged state. The six N-terminal domains are arranged in three pairs, stabilized by coiled-coil segments and forming an additional channel for substrate sensing and entry into the AAA+ ring. Sequence conservation analysis and proteolysis assays confirm that this architecture is required for the degradation of both folded and unfolded substrates in bacteria.
中文翻译:

嗜热栖热菌全长 Lon 蛋白酶的冷冻电镜结构
在细菌中,Lon 是一种大型六聚体 ATP 依赖性蛋白酶,其目标是错误折叠和折叠的底物,其中一些底物参与细胞分裂和细胞应激的生存。 Lon 的 N 端结构域有助于底物识别,但这些结构域如何赋予这种活性仍不清楚。在这里,我们以 3.9 Å 分辨率报道了嗜热栖热菌Lon 蛋白酶在底物接合状态下的全长结构。六个 N 端结构域排列成三对,由卷曲线圈段稳定,并形成用于底物感测和进入 AAA+ 环的附加通道。序列保守分析和蛋白水解测定证实,这种结构是细菌中折叠和未折叠底物降解所必需的。
更新日期:2021-11-08
中文翻译:

嗜热栖热菌全长 Lon 蛋白酶的冷冻电镜结构
在细菌中,Lon 是一种大型六聚体 ATP 依赖性蛋白酶,其目标是错误折叠和折叠的底物,其中一些底物参与细胞分裂和细胞应激的生存。 Lon 的 N 端结构域有助于底物识别,但这些结构域如何赋予这种活性仍不清楚。在这里,我们以 3.9 Å 分辨率报道了嗜热栖热菌Lon 蛋白酶在底物接合状态下的全长结构。六个 N 端结构域排列成三对,由卷曲线圈段稳定,并形成用于底物感测和进入 AAA+ 环的附加通道。序列保守分析和蛋白水解测定证实,这种结构是细菌中折叠和未折叠底物降解所必需的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号