当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-09-30 , DOI: 10.1021/acs.jmedchem.1c01215 Murugaiah A M Subbaiah 1 , Nicholas A Meanwell 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-09-30 , DOI: 10.1021/acs.jmedchem.1c01215 Murugaiah A M Subbaiah 1 , Nicholas A Meanwell 2
Affiliation
|
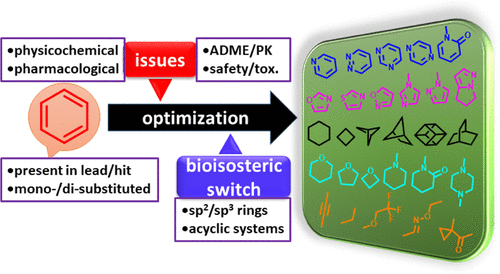
The benzene moiety is the most prevalent ring system in marketed drugs, underscoring its historic popularity in drug design either as a pharmacophore or as a scaffold that projects pharmacophoric elements. However, introspective analyses of medicinal chemistry practices at the beginning of the 21st century highlighted the indiscriminate deployment of phenyl rings as an important contributor to the poor physicochemical properties of advanced molecules, which limited their prospects of being developed into effective drugs. This Perspective deliberates on the design and applications of bioisosteric replacements for a phenyl ring that have provided practical solutions to a range of developability problems frequently encountered in lead optimization campaigns. While the effect of phenyl ring replacements on compound properties is contextual in nature, bioisosteric substitution can lead to enhanced potency, solubility, and metabolic stability while reducing lipophilicity, plasma protein binding, phospholipidosis potential, and inhibition of cytochrome P450 enzymes and the hERG channel.
中文翻译:

苯基环的生物电子等排体:铅优化和药物设计的最新战略应用
苯部分是市售药物中最普遍的环系统,突显了其作为药效团或投射药效团元素的支架在药物设计中的历史性流行。然而,21世纪初对药物化学实践的内省分析强调了苯环的不加选择是导致先进分子物理化学性质差的重要原因,这限制了它们被开发成有效药物的前景。该观点探讨了苯环的生物等排替代物的设计和应用,这些替代物为铅优化活动中经常遇到的一系列可开发性问题提供了实用的解决方案。虽然苯环取代对化合物性质的影响本质上是上下文相关的,
更新日期:2021-10-14
中文翻译:

苯基环的生物电子等排体:铅优化和药物设计的最新战略应用
苯部分是市售药物中最普遍的环系统,突显了其作为药效团或投射药效团元素的支架在药物设计中的历史性流行。然而,21世纪初对药物化学实践的内省分析强调了苯环的不加选择是导致先进分子物理化学性质差的重要原因,这限制了它们被开发成有效药物的前景。该观点探讨了苯环的生物等排替代物的设计和应用,这些替代物为铅优化活动中经常遇到的一系列可开发性问题提供了实用的解决方案。虽然苯环取代对化合物性质的影响本质上是上下文相关的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号