Redox Biology ( IF 10.7 ) Pub Date : 2021-09-29 , DOI: 10.1016/j.redox.2021.102156 Zhuqing Li 1 , Qi Li 1 , Li Wang 2 , Chao Li 2 , Mengping Xu 2 , Yajun Duan 3 , Likun Ma 4 , Tingting Li 5 , Qiao Chen 5 , Yilin Wang 5 , Yanxin Wang 5 , Jiaxin Feng 5 , Xuemei Yin 5 , Xiaolin Wang 6 , Jihong Han 7 , Chengzhi Lu 8
|
Objective
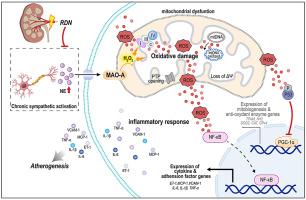
The disruption of mitochondrial redox homeostasis in endothelial cells (ECs) can cause chronic inflammation, a substantial contributor to the development of atherosclerosis. Chronic sympathetic hyperactivity can enhance oxidative stress to induce endothelial dysfunction. We determined if renal denervation (RDN), the strategy reducing sympathetic tone, can protect ECs by ameliorating mitochondrial reactive oxygen species (ROS)-induced inflammation to reduce atherosclerosis.
Methods and results
ApoE deficient (ApoE−/-) mice were conducted RDN or sham operation before 20-week high-fat diet feeding. Atherosclerosis, EC phenotype and mitochondrial morphology were determined. In vitro, human arterial ECs were treated with norepinephrine to determine the mechanisms for RDN-inhibited endothelial inflammation. RDN reduced atherosclerosis, EC mitochondrial oxidative stress and inflammation. Mechanistically, the chronic sympathetic hyperactivity increased circulating norepinephrine and mitochondrial monoamine oxidase A (MAO-A) activity. MAO-A activation-impaired mitochondrial homeostasis resulted in ROS accumulation and NF-κB activation, thereby enhancing expression of atherogenic and proinflammatory molecules in ECs. It also suppressed mitochondrial function regulator PGC-1α, with involvement of NF-κB and oxidative stress. Inactivation of MAO-A by RDN disrupted the positive-feedback regulation between mitochondrial dysfunction and inflammation, thereby inhibiting EC atheroprone phenotypic alterations and atherosclerosis.
Conclusions
The interplay between MAO-A-induced mitochondrial oxidative stress and inflammation in ECs is a key driver in atherogenesis, and it can be reduced by RDN.
中文翻译:

通过肾去神经支配靶向线粒体炎症循环可减少动脉粥样硬化内皮表型和动脉粥样硬化
客观的
内皮细胞 (EC) 中线粒体氧化还原稳态的破坏可导致慢性炎症,这是动脉粥样硬化发展的重要因素。慢性交感神经过度活跃可增强氧化应激,诱发内皮功能障碍。我们确定了肾去神经支配 (RDN),降低交感神经张力的策略,是否可以通过改善线粒体活性氧 (ROS) 诱导的炎症来减少动脉粥样硬化来保护 EC。
方法和结果
ApoE 缺陷 (ApoE -/- ) 小鼠在 20 周高脂肪饮食喂养之前进行 RDN 或假手术。测定了动脉粥样硬化、EC 表型和线粒体形态。体外,用去甲肾上腺素处理人动脉内皮细胞以确定 RDN 抑制内皮炎症的机制。RDN 减少动脉粥样硬化、EC 线粒体氧化应激和炎症。从机制上讲,慢性交感神经过度活跃会增加循环去甲肾上腺素和线粒体单胺氧化酶 A (MAO-A) 的活性。MAO-A 激活受损的线粒体稳态导致 ROS 积累和 NF-κB 激活,从而增强内皮细胞中致动脉粥样硬化和促炎分子的表达。它还抑制线粒体功能调节剂 PGC-1α,涉及 NF-κB 和氧化应激。RDN 对 MAO-A 的灭活破坏了线粒体功能障碍和炎症之间的正反馈调节,从而抑制了 EC 动脉粥样硬化表型改变和动脉粥样硬化。
结论
MAO-A 诱导的线粒体氧化应激与 ECs 炎症之间的相互作用是动脉粥样硬化的关键驱动因素,它可以通过 RDN 减少。











































 京公网安备 11010802027423号
京公网安备 11010802027423号