当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Reductive Carboxylation and Amidation Reactions
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-09-29 , DOI: 10.1021/acs.accounts.1c00480 Andreu Tortajada 1, 2 , Marino Börjesson 1, 2 , Ruben Martin 1, 3
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-09-29 , DOI: 10.1021/acs.accounts.1c00480 Andreu Tortajada 1, 2 , Marino Börjesson 1, 2 , Ruben Martin 1, 3
Affiliation
|
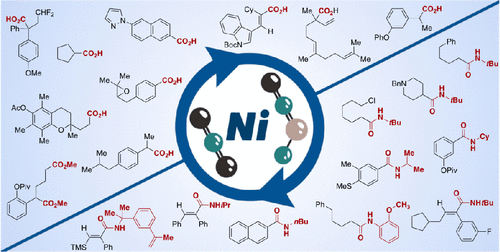
The ubiquity and importance of carboxylic acids and amides in peptides, pharmaceuticals, agrochemicals, and synthetic materials has challenged chemists to design de novo catalytic carboxylation and amidation protocols. They represent a powerful alternative to canonical oxidation of alcohols and aldehydes, hydrolysis of nitriles, transamidation reactions, or condensation techniques for the synthesis of these functional groups. Among various scenarios, the recent years have witnessed considerable advances in Ni-catalyzed reductive carboxylation and amidation reactions utilizing carbon dioxide and isocyanate counterparts. This Account aims to highlight the progress made in this arena with a historical perspective, with particular emphasis on the methodologies that have emanated from our laboratories without losing sight of the underlying principles by which these reactions operate, with the ultimate goal of allowing the transition from comprehension to prediction in this exciting field.
中文翻译:

镍催化还原羧化和酰胺化反应
羧酸和酰胺在肽、药物、农用化学品和合成材料中的普遍性和重要性已经挑战了化学家从头设计催化羧化和酰胺化方案。它们代表了醇和醛的经典氧化、腈的水解、转酰胺反应或用于合成这些官能团的缩合技术的强大替代方案。在各种情况下,近年来,利用二氧化碳和异氰酸酯对应物的镍催化还原羧化和酰胺化反应取得了长足的进步。本报告旨在从历史的角度突出这一领域取得的进展,特别强调从我们的实验室产生的方法,同时又不忽视这些反应运作的基本原则,最终目标是允许从在这个令人兴奋的领域中对预测的理解。
更新日期:2021-10-19
中文翻译:

镍催化还原羧化和酰胺化反应
羧酸和酰胺在肽、药物、农用化学品和合成材料中的普遍性和重要性已经挑战了化学家从头设计催化羧化和酰胺化方案。它们代表了醇和醛的经典氧化、腈的水解、转酰胺反应或用于合成这些官能团的缩合技术的强大替代方案。在各种情况下,近年来,利用二氧化碳和异氰酸酯对应物的镍催化还原羧化和酰胺化反应取得了长足的进步。本报告旨在从历史的角度突出这一领域取得的进展,特别强调从我们的实验室产生的方法,同时又不忽视这些反应运作的基本原则,最终目标是允许从在这个令人兴奋的领域中对预测的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号