Journal of Controlled Release ( IF 10.5 ) Pub Date : 2021-09-29 , DOI: 10.1016/j.jconrel.2021.09.035 Matthew Drayton 1 , Morgan A Alford 2 , Daniel Pletzer 3 , Evan F Haney 4 , Yoan Machado 5 , Haiming D Luo 1 , Christopher M Overall 6 , Jayachandran N Kizhakkedathu 7 , Robert E W Hancock 4 , Suzana K Straus 8
|
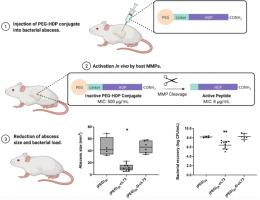
Host defense peptides (HDPs) have been the subject of great interest for the treatment of multidrug-resistant bacterial infections due to their multimodal activity and low induction of resistance. However, aggregation, toxicity, and short biological half-life have limited their applicability for clinical treatment. Many methods have been explored to alleviate these issues, such as polymer (e.g., polyethylene glycol (PEG)) conjugation, but these are often accompanied by reductions in the activity of the HDP. Here, we detail the design of a novel PEG-HDP conjugate incorporating an enzymatic cleavage sequence targeting matrix metalloproteinases (MMPs) that accumulate at sites of inflammation and infection. Addition of the cleavage sequence onto either the N- or the C-terminal region of the parent peptide (peptide 73, a derivative of the HDP aurein 2.2) was explored to determine the location for optimal antimicrobial activity following MMP cleavage; furthermore, the susceptibility of the peptide to MMP cleavage after conjugation to 2 kDa or 5 kDa PEG was examined. The top candidate, L73, utilized an N-terminal cleavage site that was subsequently conjugated to a 2 kDa PEG polymer. Both L73 and the conjugate exhibited no antimicrobial activity in vitro until cleaved by purified MMP, which liberated a peptide fragment with 16- or 63-fold improved activity, respectively, corresponding to a minimum inhibitory concentration (MIC) of 8 μg/mL, comparable to that of peptide 73 (4 μg/mL). Furthermore, PEG conjugation improved the blood compatibility and reduced the aggregation tendency of the HDP in vitro, indicating enhanced biocompatibility. When administered as a single subcutaneous dose (~3.6 mg, or a peptide concentration of 142 mg/kg) in a mouse abscess model of high-density methicillin-resistant Staphylococcus aureus (MRSA) infection, the conjugate displayed strong activity, reducing abscess size and bacterial load by 73.3% and 58-fold, respectively. This activity was completely lost when the cleavage site was rendered resistant to MMPs by the substitution of two d-amino acids, supporting the hypothesis that antimicrobial activity was dependent on cleavage by MMPs, which were shown here to increasingly accumulate at the abscess site up to 18 h post infection. Finally, the conjugate displayed biocompatibility in vivo, with no identifiable toxicity or aggregation.
中文翻译:

酶促释放聚乙二醇 - 宿主防御肽偶联物,具有更高的活性和生物相容性
宿主防御肽 (HDP) 由于其多模式活性和低耐药性诱导而成为治疗多药耐药细菌感染的重要主题。然而,聚集、毒性和短的生物半衰期限制了它们在临床治疗中的适用性。已经探索了许多方法来缓解这些问题,例如聚合物(例如, 聚乙二醇 (PEG)) 共轭,但这些通常伴随着 HDP 活性的降低。在这里,我们详细介绍了一种新型 PEG-HDP 偶联物的设计,该偶联物结合了靶向在炎症和感染部位积聚的基质金属蛋白酶 (MMP) 的酶促裂解序列。探索将切割序列添加到亲本肽(肽 73,HDP 金黄色素 2.2 的衍生物)的 N 端或 C 端区域以确定 MMP 切割后最佳抗菌活性的位置;此外,检测了与 2 kDa 或 5 kDa PEG 缀合后肽对 MMP 裂解的敏感性。最重要的候选者 L73 利用 N 端切割位点,随后与 2 kDa PEG 聚合物缀合。在体外直到被纯化的 MMP 裂解,释放出的肽片段的活性分别提高了 16 或 63 倍,对应的最小抑制浓度 (MIC) 为 8 μg/mL,与肽 73 (4 μg/mL) 相当。毫升)。此外,PEG结合改善了血液相容性并降低了体外HDP的聚集趋势,表明生物相容性增强。在高密度耐甲氧西林金黄色葡萄球菌的小鼠脓肿模型中以单次皮下剂量(~3.6 mg,或肽浓度为 142 mg/kg)给药时(MRSA) 感染,缀合物显示出强大的活性,分别将脓肿大小和细菌负荷减少 73.3% 和 58 倍。当切割位点通过替换两个d-氨基酸而对 MMP 产生抗性时,这种活性完全丧失,支持抗菌活性依赖于 MMP 切割的假设,此处显示 MMP 在脓肿部位积累越来越多感染后 18 小时。最后,该偶联物在体内表现出生物相容性,没有可识别的毒性或聚集。











































 京公网安备 11010802027423号
京公网安备 11010802027423号