Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of amino acid additives on protein solubility – insights from desorption and direct electrospray ionization mass spectrometry
Analyst ( IF 3.6 ) Pub Date : 2021-09-27 , DOI: 10.1039/d1an01392k Roshan Javanshad 1 , Andre R Venter 1
Analyst ( IF 3.6 ) Pub Date : 2021-09-27 , DOI: 10.1039/d1an01392k Roshan Javanshad 1 , Andre R Venter 1
Affiliation
|
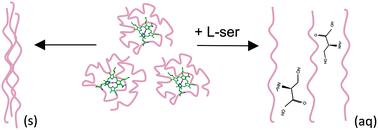
Naturally occurring amino acids have been broadly used as additives to improve protein solubility and inhibit aggregation. In this study, improvements in protein signal intensity obtained with the addition of L-serine, and structural analogs, to the desorption electrospray ionization mass spectrometry (DESI-MS) spray solvent were measured. The results were interpreted at the hand of proposed mechanisms of solution additive effects on protein solubility and dissolution. DESI-MS allows for these processes to be studied efficiently using dilute concentrations of additives and small amounts of proteins, advantages that represent real benefits compared to classical methods of studying protein stability and aggregation. We show that serine significantly increases the protein signal in DESI-MS when native proteins are undergoing unfolding during the dissolution process with an acidic solvent system (p-value = 0.0001), or with ammonium bicarbonate under denaturing conditions for proteins with high isoelectric points (p-value = 0.001). We establish that a similar increase in the protein signal cannot be observed with direct ESI-MS, and the observed increase is therefore not related to ionization processes or changes in the physical properties of the bulk solution. The importance of the presence of serine during protein conformational changes while undergoing dissolution is demonstrated through comparisons between the analyses of proteins deposited in native or unfolded states and by using native state-preserving and denaturing desorption solvents. We hypothesize that direct, non-covalent interactions involving all three functional groups of serine are involved in the beneficial effect on protein solubility and dissolution. Supporting evidence for a direct interaction include a reduction in efficacy with D-serine or the racemic mixture, indicating a non-bulk-solution physical property effect; insensitivity to the sample surface type or relative placement of serine addition; and a reduction in efficacy with any modifications to the serine structure, most notably the carboxyl functional group. An alternative hypothesis, also supported by some of our observations, could involve the role of serine clusters in the mechanism of solubility enhancement. Our study demonstrates the capability of DESI-MS together with complementary ESI-MS experiments as a novel tool for understanding protein solubility and dissolution and investigating the mechanism of action for solubility-enhancing additives.
中文翻译:

氨基酸添加剂对蛋白质溶解度的影响——来自解吸和直接电喷雾电离质谱的见解
天然存在的氨基酸已被广泛用作添加剂以提高蛋白质溶解度和抑制聚集。在这项研究中,通过添加L获得的蛋白质信号强度的改善测量解吸电喷雾电离质谱 (DESI-MS) 喷雾溶剂的丝氨酸和结构类似物。根据溶液加成效应对蛋白质溶解度和溶解的拟议机制对结果进行了解释。DESI-MS 允许使用稀释浓度的添加剂和少量蛋白质有效地研究这些过程,与研究蛋白质稳定性和聚集的经典方法相比,这些优势代表了真正的好处。我们表明,当天然蛋白质在溶解过程中使用酸性溶剂系统(p值 = 0.0001)或在具有高等电点的蛋白质变性条件下使用碳酸氢铵进行解折叠时,丝氨酸显着增加了 DESI-MS 中的蛋白质信号(p值 = 0.001)。我们确定使用直接 ESI-MS 无法观察到蛋白质信号的类似增加,因此观察到的增加与电离过程或本体溶液的物理特性变化无关。通过对以天然或未折叠状态沉积的蛋白质的分析与使用天然状态保存和变性解吸溶剂进行比较,证明了在溶解过程中蛋白质构象变化过程中丝氨酸的存在的重要性。我们假设涉及丝氨酸所有三个官能团的直接、非共价相互作用参与了对蛋白质溶解度和溶解度的有益影响。支持直接相互作用的证据包括与D 的疗效降低-丝氨酸或外消旋混合物,表明非散装溶液的物理性质影响;对样品表面类型或丝氨酸添加的相对位置不敏感;对丝氨酸结构(最显着的是羧基官能团)进行任何修改都会降低功效。另一种假设也得到了我们的一些观察结果的支持,可能涉及丝氨酸簇在溶解度增强机制中的作用。我们的研究证明了 DESI-MS 与互补的 ESI-MS 实验一起作为了解蛋白质溶解度和溶解度以及研究增溶添加剂作用机制的新工具的能力。
更新日期:2021-09-29
中文翻译:

氨基酸添加剂对蛋白质溶解度的影响——来自解吸和直接电喷雾电离质谱的见解
天然存在的氨基酸已被广泛用作添加剂以提高蛋白质溶解度和抑制聚集。在这项研究中,通过添加L获得的蛋白质信号强度的改善测量解吸电喷雾电离质谱 (DESI-MS) 喷雾溶剂的丝氨酸和结构类似物。根据溶液加成效应对蛋白质溶解度和溶解的拟议机制对结果进行了解释。DESI-MS 允许使用稀释浓度的添加剂和少量蛋白质有效地研究这些过程,与研究蛋白质稳定性和聚集的经典方法相比,这些优势代表了真正的好处。我们表明,当天然蛋白质在溶解过程中使用酸性溶剂系统(p值 = 0.0001)或在具有高等电点的蛋白质变性条件下使用碳酸氢铵进行解折叠时,丝氨酸显着增加了 DESI-MS 中的蛋白质信号(p值 = 0.001)。我们确定使用直接 ESI-MS 无法观察到蛋白质信号的类似增加,因此观察到的增加与电离过程或本体溶液的物理特性变化无关。通过对以天然或未折叠状态沉积的蛋白质的分析与使用天然状态保存和变性解吸溶剂进行比较,证明了在溶解过程中蛋白质构象变化过程中丝氨酸的存在的重要性。我们假设涉及丝氨酸所有三个官能团的直接、非共价相互作用参与了对蛋白质溶解度和溶解度的有益影响。支持直接相互作用的证据包括与D 的疗效降低-丝氨酸或外消旋混合物,表明非散装溶液的物理性质影响;对样品表面类型或丝氨酸添加的相对位置不敏感;对丝氨酸结构(最显着的是羧基官能团)进行任何修改都会降低功效。另一种假设也得到了我们的一些观察结果的支持,可能涉及丝氨酸簇在溶解度增强机制中的作用。我们的研究证明了 DESI-MS 与互补的 ESI-MS 实验一起作为了解蛋白质溶解度和溶解度以及研究增溶添加剂作用机制的新工具的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号