Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A multiplexed, automated immuno-matrix assisted laser desorption/ionization mass spectrometry assay for simultaneous and precise quantitation of PTEN and p110α in cell lines and tumor tissues
Analyst ( IF 3.6 ) Pub Date : 2021-09-21 , DOI: 10.1039/d1an00165e Bjoern C Froehlich 1, 2 , Robert Popp 1 , Constance A Sobsey 3 , Sahar Ibrahim 3 , Andre LeBlanc 3 , Yassene Mohammed 1, 4, 5 , Marguerite Buchanan 6 , Adriana Aguilar-Mahecha 6 , Oliver Pötz 7, 8 , Michael X Chen 9 , Alan Spatz 6 , Mark Basik 6 , Gerald Batist 6, 10 , René P Zahedi 3, 5 , Christoph H Borchers 1, 2, 3, 5, 10
Analyst ( IF 3.6 ) Pub Date : 2021-09-21 , DOI: 10.1039/d1an00165e Bjoern C Froehlich 1, 2 , Robert Popp 1 , Constance A Sobsey 3 , Sahar Ibrahim 3 , Andre LeBlanc 3 , Yassene Mohammed 1, 4, 5 , Marguerite Buchanan 6 , Adriana Aguilar-Mahecha 6 , Oliver Pötz 7, 8 , Michael X Chen 9 , Alan Spatz 6 , Mark Basik 6 , Gerald Batist 6, 10 , René P Zahedi 3, 5 , Christoph H Borchers 1, 2, 3, 5, 10
Affiliation
|
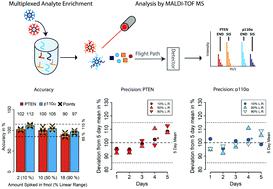
The PI3-kinase/AKT/mTOR pathway plays a central role in cancer signaling. While p110α is the catalytic α-subunit of PI3-kinase and a major drug target, PTEN is the main negative regulator of the PI3-kinase/AKT/mTOR pathway. PTEN is often down-regulated in cancer, and there are conflicting data on PTEN's role as breast cancer biomarker. PTEN and p110α protein expression in tumors is commonly analyzed by immunohistochemistry, which suffers from poor multiplexing capacity, poor standardization, and antibody crossreactivity, and which provides only semi-quantitative data. Here, we present an automated, and standardized immuno-matrix-assisted laser desorption/ionization mass spectrometry (iMALDI) assay that allows precise and multiplexed quantitation of PTEN and p110α concentrations, without the limitations of immunohistochemistry. Our iMALDI assay only requires a low-cost benchtop MALDI-TOF mass spectrometer, which simplifies clinical translation. We validated our assay's precision and accuracy, with simultaneous enrichment of both target proteins not significantly affecting the precision and accuracy of the quantitation when compared to the PTEN- and p110α-singleplex iMALDI assays (<15% difference). The multiplexed assay's linear range is from 0.6–20 fmol with accuracies of 90–112% for both target proteins, and the assay is free of matrix-related interferences. The inter-day reproducibility over 5-days was high, with an overall CV of 9%. PTEN and p110α protein concentrations can be quantified down to 1.4 fmol and 0.6 fmol per 10 μg of total tumor protein, respectively, in various tumor tissue samples, including fresh-frozen breast tumors and colorectal cancer liver metastases, and patient-derived xenograft (PDX) tumors.
中文翻译:

多路自动化免疫基质辅助激光解吸/电离质谱分析,用于同时精确定量细胞系和肿瘤组织中的 PTEN 和 p110α
PI3-激酶/AKT/mTOR 通路在癌症信号传导中起着核心作用。p110α 是 PI3-激酶的催化 α-亚基和主要药物靶点,而 PTEN 是 PI3-激酶/AKT/mTOR 通路的主要负调节因子。PTEN 在癌症中经常被下调,关于 PTEN 作为乳腺癌生物标志物的作用存在相互矛盾的数据。肿瘤中 PTEN 和 p110α 蛋白表达通常通过免疫组织化学分析,其存在多重能力差、标准化差和抗体交叉反应性差,并且仅提供半定量数据。在这里,我们提出了一种自动化、标准化的免疫基质辅助激光解吸/电离质谱 (iMALDI) 分析,它允许对 PTEN 和 p110α 浓度进行精确和多重定量,而不受免疫组织化学的限制。我们的 iMALDI 检测只需要一台低成本的台式 MALDI-TOF 质谱仪,从而简化了临床转化。我们验证了我们的检测的精确度和准确度,与 PTEN 和 p110α 单链 iMALDI 检测相比,同时富集两种目标蛋白不会显着影响定量的精确度和准确度(差异 <15%)。多重检测的线性范围为 0.6-20 fmol,两种目标蛋白的准确度为 90-112%,并且该检测没有基质相关干扰。5 天的日间重现性很高,总体 CV 为 9%。在各种肿瘤组织样本中,PTEN 和 p110α 蛋白浓度可以分别量化为每 10 μg 总肿瘤蛋白 1.4 fmol 和 0.6 fmol,
更新日期:2021-09-29
中文翻译:

多路自动化免疫基质辅助激光解吸/电离质谱分析,用于同时精确定量细胞系和肿瘤组织中的 PTEN 和 p110α
PI3-激酶/AKT/mTOR 通路在癌症信号传导中起着核心作用。p110α 是 PI3-激酶的催化 α-亚基和主要药物靶点,而 PTEN 是 PI3-激酶/AKT/mTOR 通路的主要负调节因子。PTEN 在癌症中经常被下调,关于 PTEN 作为乳腺癌生物标志物的作用存在相互矛盾的数据。肿瘤中 PTEN 和 p110α 蛋白表达通常通过免疫组织化学分析,其存在多重能力差、标准化差和抗体交叉反应性差,并且仅提供半定量数据。在这里,我们提出了一种自动化、标准化的免疫基质辅助激光解吸/电离质谱 (iMALDI) 分析,它允许对 PTEN 和 p110α 浓度进行精确和多重定量,而不受免疫组织化学的限制。我们的 iMALDI 检测只需要一台低成本的台式 MALDI-TOF 质谱仪,从而简化了临床转化。我们验证了我们的检测的精确度和准确度,与 PTEN 和 p110α 单链 iMALDI 检测相比,同时富集两种目标蛋白不会显着影响定量的精确度和准确度(差异 <15%)。多重检测的线性范围为 0.6-20 fmol,两种目标蛋白的准确度为 90-112%,并且该检测没有基质相关干扰。5 天的日间重现性很高,总体 CV 为 9%。在各种肿瘤组织样本中,PTEN 和 p110α 蛋白浓度可以分别量化为每 10 μg 总肿瘤蛋白 1.4 fmol 和 0.6 fmol,











































 京公网安备 11010802027423号
京公网安备 11010802027423号