Clinical Gastroenterology and Hepatology ( IF 11.6 ) Pub Date : 2021-09-29 , DOI: 10.1016/j.cgh.2021.09.028 David A Schwartz 1 , Laurent Peyrin-Biroulet 2 , Karen Lasch 3 , Shashi Adsul 4 , Silvio Danese 5
|
Background & Aims
Fistulizing Crohn’s disease (CD) is challenging to treat. We report results from ENTERPRISE, a randomized, double-blind, phase 4 trial evaluating 2 vedolizumab intravenous dosing regimens in patients with fistulizing CD.
Methods
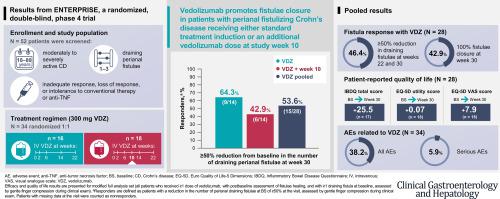
Patients with moderately to severely active CD and 1–3 active perianal fistulae (identified on magnetic resonance imaging [MRI]) received vedolizumab 300 mg intravenously at weeks 0, 2, 6, 14, and 22 (VDZ) or the same regimen plus an additional vedolizumab dose at week 10 (VDZ + wk10). Reduction from baseline in draining perianal fistulae and disease activity, MRI assessments, health-related quality of life (HRQoL), and safety were evaluated. Enrollment was stopped prematurely because of recruitment challenges; analyses are descriptive.
Results
Of 32 patients with ≥1 active fistulae at baseline per MRI and postbaseline fistulae healing assessment, 28 (14 per dosing regimen) had ≥1 draining fistulae at baseline (assessed by gentle finger compression during clinical exam). Rapid and sustained fistula closure was observed; 53.6% (VDZ, 64.3%; VDZ + wk10, 42.9%) and 42.9% (VDZ, 50.0%; VDZ + wk10, 35.7%) of patients achieved ≥50% decrease in draining fistulae and 100% fistulae closure, respectively, at week 30. Mean (standard deviation) CD and Perianal Disease Activity Index scores decreased by 51.1 (78.3) and 4.1 (3.3), respectively, at week 30. HRQoL improved throughout the study. No new safety signals were observed.
Conclusions
Sustained improvements in fistulizing CD were seen with both vedolizumab regimens. An additional dose at week 10 does not appear to alter treatment outcomes. Safety profile was consistent with other vedolizumab studies. ClinicalTrials.gov no: NCT02630966; EudraCT: 2015-000852-12.
中文翻译:

2 种 Vedolizumab 静脉给药方案治疗肛周造瘘克罗恩病的疗效和安全性:企业研究
背景与目标
造瘘克罗恩病 (CD) 的治疗具有挑战性。我们报告了 ENTERPRISE 的结果,这是一项随机、双盲、4 期试验,评估 2 种 vedolizumab 静脉给药方案在瘘管 CD 患者中的作用。
方法
中度至重度活动性 CD 和 1-3 个活动性肛周瘘管(通过磁共振成像 [MRI] 确定)的患者在第 0、2、6、14 和 22 周(VDZ)静脉内接受 300 mg vedolizumab 或相同方案加在第 10 周(VDZ + wk10)额外的 vedolizumab 剂量。评估了引流肛周瘘和疾病活动、MRI 评估、健康相关生活质量 (HRQoL) 和安全性从基线的减少。由于招聘方面的挑战,过早停止招生;分析是描述性的。
结果
在每次 MRI 和基线后瘘管愈合评估时,32 名患者在基线时有 ≥1 个活动性瘘管,其中 28 名(每个给药方案 14 个)在基线时有 ≥1 个引流瘘管(通过临床检查期间轻柔的手指按压来评估)。观察到快速和持续的瘘管闭合;53.6% (VDZ, 64.3%; VDZ + wk10, 42.9%) 和 42.9% (VDZ, 50.0%; VDZ + wk10, 35.7%) 的患者分别在第 30 周。平均(标准差)CD 和肛周疾病活动指数评分在第 30 周分别降低了 51.1 (78.3) 和 4.1 (3.3)。HRQoL 在整个研究过程中得到改善。没有观察到新的安全信号。
结论
两种 vedolizumab 方案均观察到瘘管 CD 的持续改善。第 10 周的额外剂量似乎不会改变治疗结果。安全性概况与其他 vedolizumab 研究一致。ClinicalTrials.gov 编号:NCT02630966;EudraCT:2015-000852-12。











































 京公网安备 11010802027423号
京公网安备 11010802027423号