当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methylglyoxal Forms Diverse Mercaptomethylimidazole Crosslinks with Thiol and Guanidine Pairs in Endogenous Metabolites and Proteins
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-09-28 , DOI: 10.1021/acschembio.1c00553 John S Coukos 1 , Raymond E Moellering 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-09-28 , DOI: 10.1021/acschembio.1c00553 John S Coukos 1 , Raymond E Moellering 1
Affiliation
|
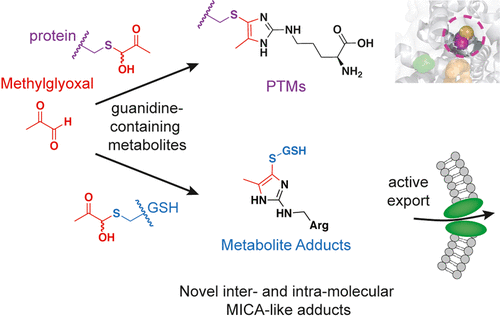
Methylglyoxal (MGO) is a reactive byproduct formed by several metabolic precursors, the most notable being triosephosphates in glycolysis. While many MGO-mediated adducts have been described, the reactivity and specific biomolecular targets of MGO remain incompletely mapped. Based on our recent discovery that MGO can form stable mercaptomethylimidazole crosslinks between cysteine and arginine (MICA) in proteins, we hypothesized that MGO may participate in myriad reactions with biologically relevant guanidines and thiols in proteins, metabolites, and perhaps other biomolecules. Herein, we performed steady-state and kinetic analyses of MGO reactivity with several model thiols, guanidines, and biguanide drugs to establish the plausible and prevalent adducts formed by MGO in proteins, peptides, and abundant cellular metabolites. We identified several novel, stable MICA metabolites that form in vitro and in cells, as well as a novel intermolecular post-translational MICA modification of surface cysteines in proteins. These data confirm that kinetic trapping of free MGO by thiols occurs rapidly and can decrease formation of more stable imidazolone (MG-H1) arginine adducts. However, reversible hemithioacetal adducts can go on to form stable MICA modifications in an inter- and intramolecular fashion with abundant or proximal guanidines, respectively. Finally, we discovered that intracellular MICA-glutathione metabolites are recognized and exported by the efflux pump MRP1, providing a parallel and perhaps complementary pathway for MGO detoxification working alongside the glyoxalase pathway. These data provide new insights into the plausible reactions involving MGO in cells and tissues, as well as several new molecular species in proteins and metabolites for further study.
中文翻译:

甲基乙二醛与内源性代谢物和蛋白质中的硫醇和胍对形成多种巯基甲基咪唑交联
甲基乙二醛 (MGO) 是由几种代谢前体形成的反应性副产物,最值得注意的是糖酵解中的磷酸丙糖。虽然已经描述了许多 MGO 介导的加合物,但 MGO 的反应性和特定的生物分子靶点仍未完全绘制出来。基于我们最近发现 MGO 可以在蛋白质中的半胱氨酸和精氨酸 (MICA) 之间形成稳定的巯基甲基咪唑交联,我们假设 MGO 可能参与与蛋白质、代谢物和其他生物分子中的生物学相关的胍和硫醇的无数反应。在这里,我们对 MGO 与几种模型硫醇、胍和双胍药物的反应性进行了稳态和动力学分析,以确定 MGO 在蛋白质、肽和丰富的细胞代谢物中形成的合理且普遍的加合物。体外和在细胞中,以及蛋白质中表面半胱氨酸的新型分子间翻译后 MICA 修饰。这些数据证实,硫醇对游离 MGO 的动力学捕获迅速发生,并且可以减少更稳定的咪唑酮 (MG-H1) 精氨酸加合物的形成。然而,可逆的半硫缩醛加合物可以继续以分子间和分子内方式形成稳定的 MICA 修饰,分别具有丰富的或近端的胍。最后,我们发现细胞内 MICA-谷胱甘肽代谢物被外排泵 MRP1 识别和输出,为与乙二醛酶途径一起工作的 MGO 解毒提供了平行且可能互补的途径。这些数据为细胞和组织中涉及 MGO 的合理反应提供了新的见解,
更新日期:2021-11-19
中文翻译:

甲基乙二醛与内源性代谢物和蛋白质中的硫醇和胍对形成多种巯基甲基咪唑交联
甲基乙二醛 (MGO) 是由几种代谢前体形成的反应性副产物,最值得注意的是糖酵解中的磷酸丙糖。虽然已经描述了许多 MGO 介导的加合物,但 MGO 的反应性和特定的生物分子靶点仍未完全绘制出来。基于我们最近发现 MGO 可以在蛋白质中的半胱氨酸和精氨酸 (MICA) 之间形成稳定的巯基甲基咪唑交联,我们假设 MGO 可能参与与蛋白质、代谢物和其他生物分子中的生物学相关的胍和硫醇的无数反应。在这里,我们对 MGO 与几种模型硫醇、胍和双胍药物的反应性进行了稳态和动力学分析,以确定 MGO 在蛋白质、肽和丰富的细胞代谢物中形成的合理且普遍的加合物。体外和在细胞中,以及蛋白质中表面半胱氨酸的新型分子间翻译后 MICA 修饰。这些数据证实,硫醇对游离 MGO 的动力学捕获迅速发生,并且可以减少更稳定的咪唑酮 (MG-H1) 精氨酸加合物的形成。然而,可逆的半硫缩醛加合物可以继续以分子间和分子内方式形成稳定的 MICA 修饰,分别具有丰富的或近端的胍。最后,我们发现细胞内 MICA-谷胱甘肽代谢物被外排泵 MRP1 识别和输出,为与乙二醛酶途径一起工作的 MGO 解毒提供了平行且可能互补的途径。这些数据为细胞和组织中涉及 MGO 的合理反应提供了新的见解,











































 京公网安备 11010802027423号
京公网安备 11010802027423号