Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-09-27 , DOI: 10.1016/j.jhazmat.2021.127368 Lingqing Wang 1 , Xiaoxiao Han 1 , Tao Liang 1 , Xiulan Yan 1 , Xiao Yang 1 , Zhiguo Pei 2 , Shuhan Tian 1 , Shengsen Wang 3 , Eder C Lima 4 , Jörg Rinklebe 5
|
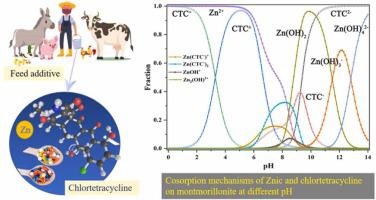
Ionic antibiotics and metals generally coexist, and their interaction can affect their sorption behaviors onto soil minerals, therefore determining their environmental hazards. This study investigated the sorption and cosorption of Zn(II) and chlortetracycline (CTC) onto montmorillonite at different solution pH (3−10) using batch experiments and extended X-ray absorption fine structure (EXAFS) analysis. The Langmuir model could reproduce well the sorption isotherms of Zn(II) and CTC. The presence of CTC/Zn(II) could promote the maximum sorption capacity (Qm) of Zn(II)/CTC, based on site energy distribution (SED) theory. Generally, Zn(II) sorption increased with pH increasing. Comparatively, CTC sorption decreased as pH increased till approximately pH 5.0, then increased continuously with pH increasing. Both CTC and Zn(II) co-existence enhanced their individual sorption in both acidic and neutral environments. The processes behind CTC and Zn(II) sorption mainly included cation exchange and surface complexation. The EXAFS data evidenced that the presence of CTC could alter the species of Zn(II) on montmorillonite via surface complexation at pH 4.5 and 7.5, with Zn-CTC complexes being the predominant species on montmorillonite at pH 7.5. At pH 9.5, Zn(II) may exist onto montmorillonite in precipitated form similar to Zn-Al hydrotalcite-like compound (HTlc) regardless of CTC presence.
中文翻译:

Zn(II) 和金霉素在蒙脱石上的共吸附:pH 效应和分子研究
离子抗生素和金属通常共存,它们的相互作用会影响它们对土壤矿物质的吸附行为,从而决定它们对环境的危害。本研究使用批量实验和扩展 X 射线吸收精细结构 (EXAFS) 分析研究了不同溶液 pH (3-10) 下 Zn(II) 和金霉素 (CTC) 在蒙脱石上的吸附和共吸附。Langmuir 模型可以很好地再现 Zn(II) 和 CTC 的吸附等温线。CTC/Zn(II) 的存在可以促进最大吸附容量 ( Q m) 的 Zn(II)/CTC,基于位点能量分布 (SED) 理论。通常,Zn(II) 吸附随着 pH 值的增加而增加。相比之下,CTC 吸附随着 pH 值的增加而降低,直到大约 pH 5.0,然后随着 pH 值的增加而不断增加。CTC 和 Zn(II) 共存增强了它们在酸性和中性环境中的单独吸附。CTC 和 Zn(II) 吸附的过程主要包括阳离子交换和表面络合。EXAFS 数据表明,CTC 的存在可以通过在 pH 4.5 和 7.5 下的表面络合改变蒙脱石上 Zn(II) 的种类,其中 Zn-CTC 配合物是 pH 7.5 时蒙脱石上的主要种类。在 pH 9.5 时,无论 CTC 是否存在,Zn(II) 都可能以类似于 Zn-Al 类水滑石化合物 (HTlc) 的沉淀形式存在于蒙脱石上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号